Figure 3.
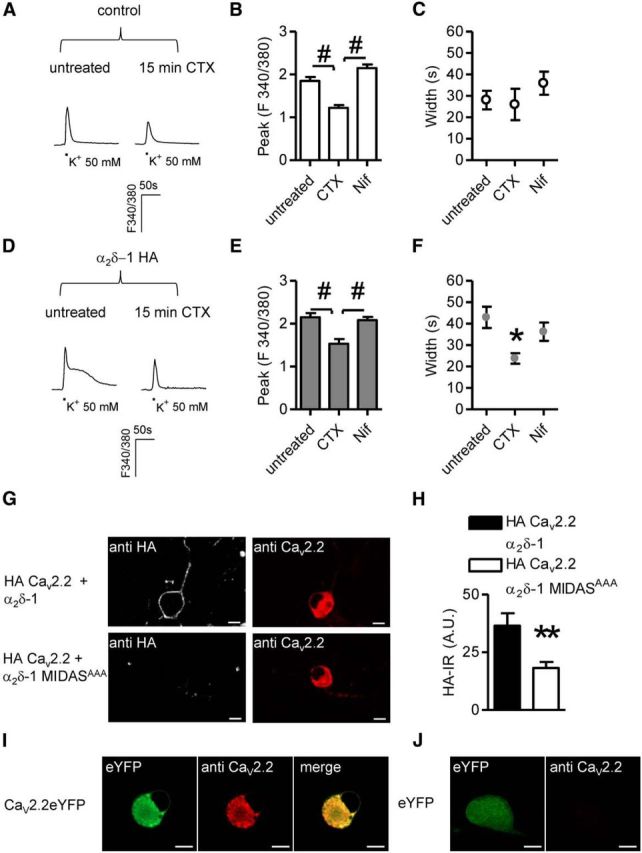
CaV2.2-mediated signaling in α2δ-1 neurons. A, Imaging of high K+-evoked Ca2+ transients in control DRG neurons pretreated for 15 min with 1 μm CTX. B, In control DRG neurons, in the absence of α2δ-1 HA overexpression CTX application (n = 7) decreased the peak amplitude of responses with respect to untreated (n = 21) and Nif (1 μm; n = 13) samples (p < 0.0001 one-way ANOVA and Bonferroni post hoc test, #p < 0.001). C, Inhibition of N- and L-type channels did not alter the width of signals imaged in control neurons (p = 0.4, one-way ANOVA). D, Examples of Ca2+ responses evoked in α2δ-1 HA overexpressing neurons in the absence (left) or presence (right) of CTX. E, CTX pretreatment (n = 12) diminished the intensity of signals in α2δ-1 HA overexpressing DRG neurons compared with responses obtained in untreated (n = 19) or Nif-treated (n = 19) DRGs (p = 0.0001 one-way ANOVA and Bonferroni post hoc test, #p < 0.001). F, Prolonged high K+-evoked Ca2+ transients were abolished following CTX pretreatment (p = 0.02 one-way ANOVA and Bonferroni post hoc test, *p < 0.05). G, Overexpression in DRG neurons of exofacially HA-tagged CaV2.2, β1B subunits and either α2δ-1 (top) or α2δ-1 MIDASAAA (bottom). Surface and intracellular detection of HA CaV2.2 proteins was performed by staining the HA epitope in nonpermeabilizing conditions and intracellular labeling with an anti CaV2.2 antibody, respectively. Scale bars, 10 μm. H, Quantification of surface HA staining in HA CaV2.2/α2δ-1/β1B (black bar; n = 32) or HA CaV2.2/α2δ-1 MIDASAAA/β1B (white bar; n = 36) transfected neurons (p = 0.02, t test). I, Labeling of overexpressed channels by CaV2.2 polyclonal antibody. DRG neurons were transfected with CaV2.2YFP and β1B cDNAs. J, CaV2.2 antibody failed to identify native subunits in eYFP overexpressing neurons indicating that the amount of protein is a limiting factor in immunocytochemistry experiments.
