Figure 1.
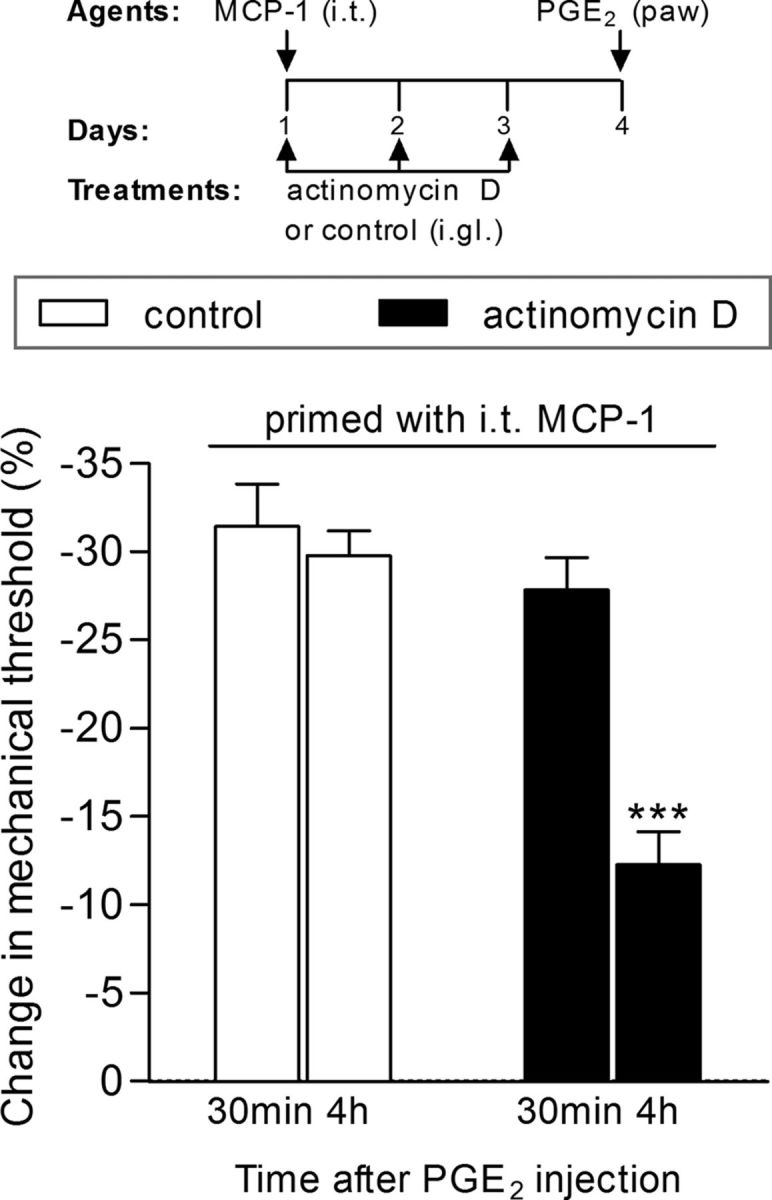
Induction of hyperalgesic priming by intrathecal (i.t.) injection of MCP-1 is dependent on gene transcription. Rats received three daily intraganglion (i.gl.) injections of vehicle (white bars) or the protein transcription inhibitor actinomycin D (10 μg, black bars). Thirty minutes after the first injection of actinomycin D, the central terminal of these nociceptors was stimulated with MCP-1 (20 ng/μl, 20 μl), injected intrathecally. No effect of actinomycin D on MCP-1-induced hyperalgesia was observed (data not shown). The injections of vehicle or actinomycin D continued, once a day, for 3 d. On the fourth day, PGE2 (100 ng) was injected intradermally into the dorsum of the hindpaw at the site of nociceptive testing to evaluate for the presence of priming. The average paw-withdrawal thresholds before injections of vehicle/MCP-1 and immediately before the injection of PGE2 (4 d later) were 110.0 ± 3.6 and 110.0 ± 2.7 g, respectively; for the groups that received actinomycin D/MCP-1, 113.3 ± 3.3 and 113.5 ± 2.2 g, respectively (t(5) = 0.0000; p = 1.0000, NS in both cases; paired Student's t test). Mechanical hyperalgesia was then evaluated by the Randall–Sellitto paw-withdrawal test 30 min and 4 h after PGE2 injection. We observed that, in the paws ipsilateral to the DRGs that received vehicle, the magnitude of hyperalgesia was still significant at the fourth hour after PGE2 injection, indicating the presence of hyperalgesic priming, while in the paws ipsilateral to the DRGs that received actinomycin D, the PGE2-induced hyperalgesia was significantly attenuated at this time point, which is compatible with the prevention of priming (***p < 0.001, when both groups are compared at the fourth hour; two-way repeated-measures ANOVA followed by Bonferroni post-test, N = 6 paws per group). The results of this experiment support the suggestion that the inhibition of transcription in the cell body prevented the induction of priming by intrathecal MCP-1.
