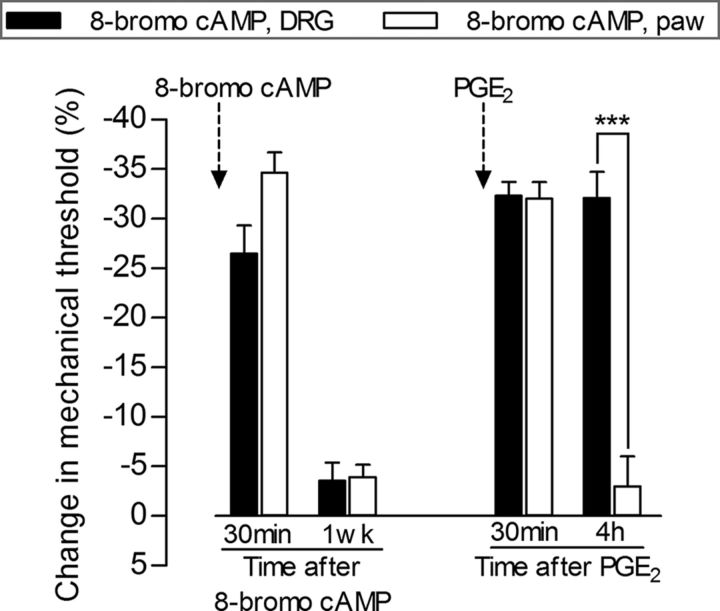
Figure 2.
Intraganglion, but not intradermal, injection of 8-bromo cAMP induces hyperalgesic priming. Rats received injection of 8-bromo cAMP in the DRG (10 μg, black bars) or intradermally in the dorsum of the hindpaw (10 μg, white bars). Robust mechanical hyperalgesia, evaluated 30 min later, by the Randall–Sellitto paw-withdrawal test, was observed in both groups (p > 0.05 when compared). One week later, a time point when the mechanical thresholds were not significantly different from pre-8-bromo cAMP baseline thresholds (data not shown), PGE2 (100 ng) was injected intradermally into the dorsum of the hindpaw. The average paw-withdrawal thresholds before the injection of 8-bromo cAMP and immediately before the injection of PGE2 (1 week later) were as follows: 120.6 ± 1.6 and 116.3 ± 2.0 g, respectively, for the DRG group (t(5) = 1.904; p = 0.1152, NS); 122.3 ± 3.0 and 116.3 ± 2.3 g, respectively, for the paw group (t(5) = 1.861; p = 0.1219, NS); no significant difference (NS) between these two pairs of values was observed (paired Student's t test). Although the mechanical hyperalgesia induced by PGE2 at the 30 min time point was not different between the groups, 4 h after injection, the PGE2 hyperalgesia was still significant in the group previously treated with intraganglion 8-bromo cAMP, but not in the group that received an injection of 8-bromo cAMP in the hindpaw (***p < 0.001, when both groups are compared at the fourth hour; two-way repeated-measures ANOVA followed by Bonferroni post-test, N = 6 paws per group), indicating that 8-bromo cAMP induces priming by acting at the level of the cell body of the nociceptor in the DRG.