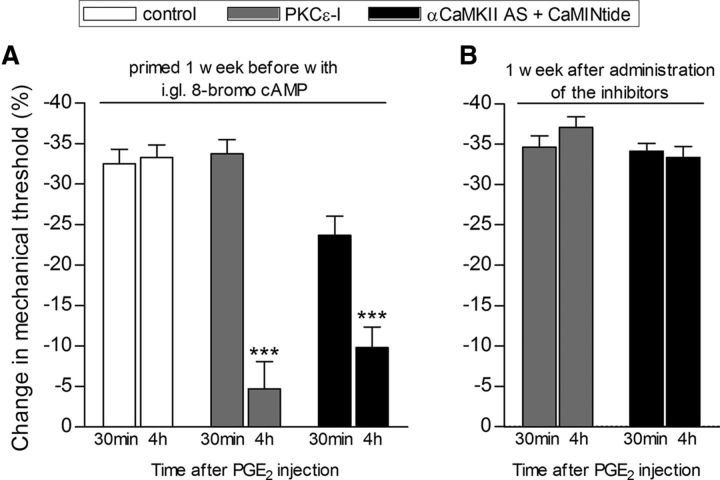
Figure 7.
Expression of hyperalgesic priming induced by intraganglion injection of 8-bromo cAMP is dependent on PKCε and αCaMKII. Rats primed with intraganglion (i.gl.) injection of 8-bromo cAMP (10 μg) 1 week prior received intradermal injection of PGE2 (100 ng) into the dorsum of the hindpaw in the presence or absence of inhibitors of the second messengers, PKCε (PKCε-I; 1 μg/5 μl, gray bars) and αCaMKII (CaMINtide; 1 μg/5 μl, black bars). Of note, in order to achieve a stronger inhibition of αCaMKII, rats were treated, for 3 consecutive days, prior to injection of the inhibitor plus PGE2, with ODN AS for αCaMKII. Mechanical nociceptive thresholds were evaluated 30 min and 4 h after PGE2, injected 5 min after the inhibitors, by the Randall–Selitto paw-withdrawal test. We observed, in both cases, significant attenuation of the hyperalgesia induced by PGE2 at the fourth hour (***p < 0.001) in the groups treated with the PKCε inhibitor or αCaMKII ODN AS plus CaM2INtide when compared with the vehicle groups (PKCε-I: F(1,10) = 29.55, p = 0.0003; αCaMKII ODN AS plus CaM2INtide: F(1,10) = 54.61, p < 0.0001, two-way repeated-measures ANOVA followed by Bonferroni post-test, N = 6 paws per group), indicating a role of PKCε and αCaMKII in the prolongation of PGE2 hyperalgesia in the i.gl. 8-bromo cAMP-induced hyperalgesic priming (A). B shows that, when PGE2 was injected again at the same site 1 week later, it produced prolonged hyperalgesia, evaluated at the fourth hour, indicating that the reversal of hyperalgesic priming induced by i.gl. 8-bromo cAMP by the inhibition of PKCε or αCaMKII is not permanent (PKCε-I: t(5) = 3.072, p = 0.2770; αCaMKII ODN AS + CaM2INtide: t(5) = 0.6532, p = 0.5424, paired Student's t test, no difference when the 30 min and 4 h time points are compared in both cases; N = 6 paws per group).