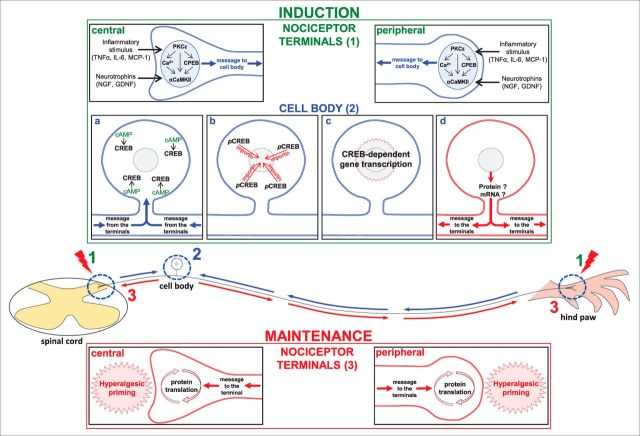
Figure 9.
Hyperalgesic priming mechanisms in the nociceptor terminals and cell body. A schematic of the nociceptor with the central terminal in the spinal cord, and the peripheral terminal in the rat hindpaw, is shown. The blue arrows represent the message triggered by stimulation in either of the terminals and directed toward the cell body, and the red arrows represent the message coming from the cell body to the terminals, where the maintenance of the primed state will take place. On the top is described the induction phase of hyperalgesic priming, separated by the events occurring in the nociceptor terminals (1) and in the cell body (2): inflammatory stimuli such as TNF-α, IL-6, or MCP-1, or the administration of neurotrophins (NGF or GDNF) in the terminals of the nociceptor (1) induce the events that lead to the development of hyperalgesic priming. Subsequent activation of PKCε stimulates CPEB, αCaMKII, or the release of calcium (Ca2+; Aley et al., 2000; Reichling and Levine, 2009; Bogen et al., 2012; Ferrari et al., 2013b), which will produce the message directed toward the cell body (Ferrari et al., 2015). a–c, There (2), activation of cAMP (a) activates the transcription factor CREB (b; pCREB), which will be transported into the nucleus by importin, where gene transcription will take place (c). d, The resulting protein or mRNA will then be transported to the terminals of the nociceptor. On the bottom, the maintenance phase of hyperalgesic priming is shown: at the terminals, ongoing protein translation, triggered by the message originated in the cell body (Bogen et al., 2012; Ferrari et al., 2013c, 2015), is responsible for the maintenance of the neuroplasticity observed in the primed state.