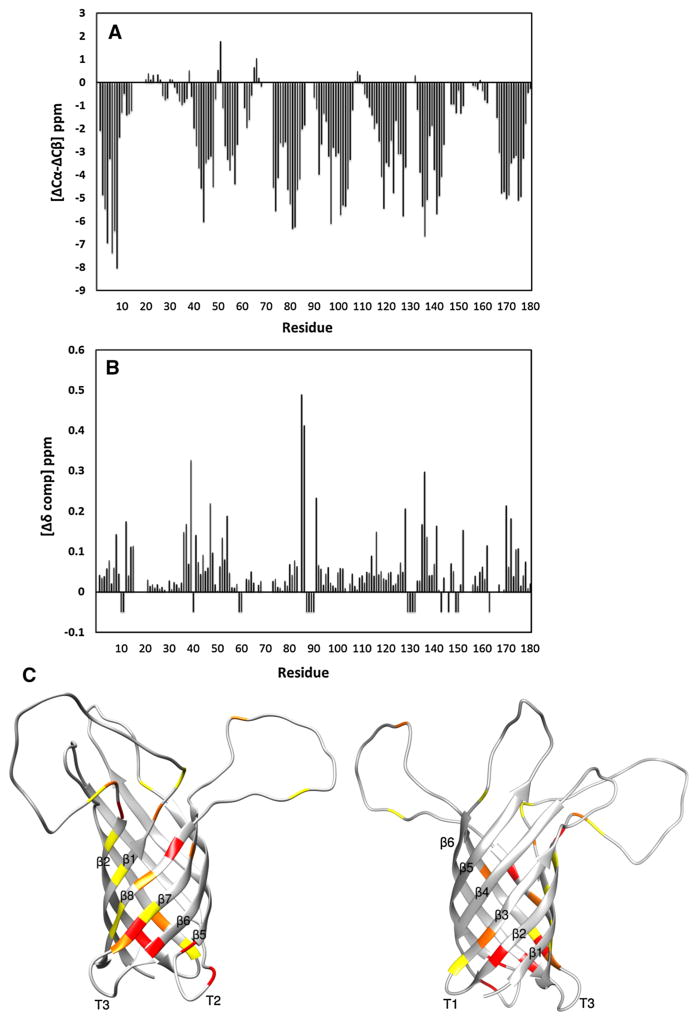
Fig. 8.
Chemical shift differences between OprH in DHPC micelles and q = 0.3 DHPC:DMPC:DHPS:DMPS (27:9:3:1) bicelles. a Three-bond averaged 13C secondary chemical shifts of 2H-,15N-,13C-labeled OprH in q = 0.3 DHPC:DMPC:DHPS:DMPS (27:9:3:1) bicelles. b Compound chemical shift differences ( ) (Mulder et al. 1999) between 2H,15N,13C-labeled OprH in micelles and q = 0.3 bicelles. Negative values indicate OprH residues unassigned in q = 0.3 bicelles. c The compound chemical shift differences of (b) are mapped onto the lowest-energy conformer of OprH in DHPC micelles (PDB code: 2LHF). Increasing chemical shift changes are depicted with increasingly more red colors. Yellow, orange, and red depict Δδ changes of 0.1–0.15, 0.15–0.2, and more than 0.2 ppm, respectively. The largest chemical shift perturbations occur near the interfacial regions of the β-strands and on the front face (left) rather than on the back face (right) of the β-barrel