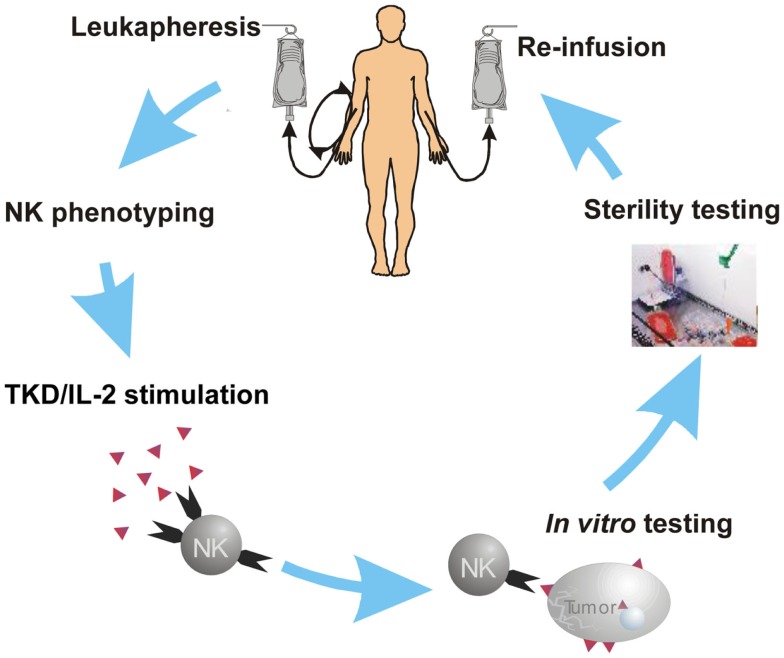
Figure 1.
Scheme of the NK cell activation in the clinical phase I trial. NSCLC patients after radiochemotherapy undergo leukapheresis and the immune phenotype (NK cell and T cell markers) is assessed by flow cytometry. Following erythrocyte depletion on a SEPAX system peripheral blood lymphocytes (PBLs) are stimulated ex vivo in a GMP laboratory with TKD/IL-2 for 3–5 days. After measuring of NK activation markers and sterility testing, the activated cells are washed and re-infused (i.v.) in the patient.