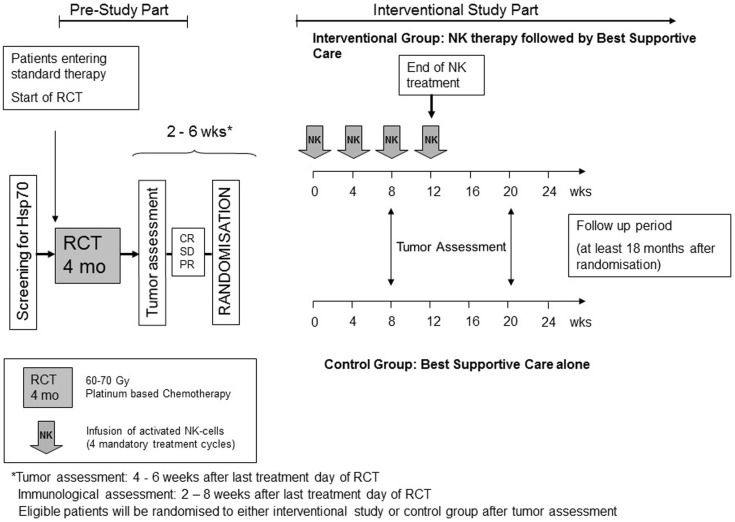
Figure 2.
Scheme of the phase II clinical trial. In the pre-study part, the Hsp70 phenotype and the stage of the tumor disease is assessed. Only Hsp70-positive NSCLC patients in stage IIIA/B who received a radiochemotherapy (RCTx) and showed at least a stable disease are randomized into the trial. The interventional group receives four cycles of ex vivo TKD/IL-2 activated NK cells on a monthly basis; the control group gets best supportive care. Tumors will be assessed in the first year every 2–3 months by CT in both groups.