Figure 1.
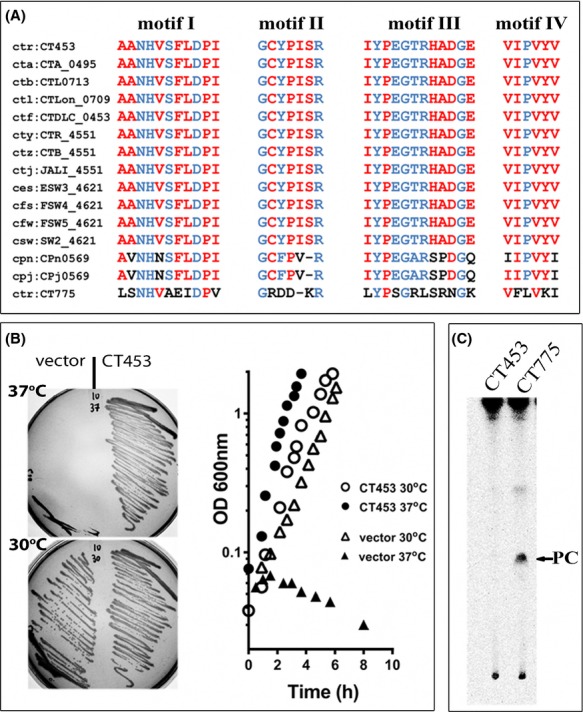
CT453 is a 1-acyl-sn-glycerol-3-phosphate acyltransferase. (A) Amino acids alignment of the predicted four conserved acyltransferase motifs of Chlamydia trachomatis D CT453 (top row, GI15605180) and some of the homolog proteins identified in other Chlamydia species and serovar, as follows: cta:CTA_0495 (Chlamydia trachomatis A/HAR-13, GI76789189); ctb:CTL0713 (Chlamydia trachomatis L2/434/Bu, GI166154667); ctl:CTLon_0709 (Chlamydia trachomatis L2b/UCH-1/proctitis, GI166155542); ctf:CTDLC_0453 (Chlamydia trachomatis D-LC,GI385244541); cty:CTR_4551 (Chlamydia trachomatis A2497, GI376282462); ctz:CTB_4551 (Chlamydia trachomatis B/TZ1A828/OT, GI237804803); ctj:JALI_4551 (Chlamydia trachomatis B/Jali20/OT, GI237802881); ces:ESW3_4621 (Chlamydia trachomatis E/SW3, GI389859025); cfs:FSW4_4621 (Chlamydia trachomatis F/SW4, GI389858149); cfw:FSW5_4621 (Chlamydia trachomatis F/SW5, GI389859901); csw:SW2_4621 (Chlamydia trachomatis Sweden2, GI386262810); cpn:CPn0569 (Chlamydia pneumoniae CWL029, GI15618480); cpj:CPj0569 (Chlamydia pneumoniae J138, GI15836100); ctr:CT775 (Chlamydia trachomatis D, GI15605508). Motifs for C. trachomatis LPCAT enzyme, CT775, are shown in the bottom row. Conserved residues defining the four motifs are shown in blue and other conserved residues are in red. (B) Rescue of the growth defect of the E. coli plsC101 mutant at 37°C by CT453 was monitored on agar plate and in liquid media. Note that the mutant strain transformed with the CT453 construct grew at 30°C and 37°C whereas the control strain only grew at 30°C. (C) Absence of a lysoPC acyltransferase activity of the CT453 enzyme was determined in reactions performed with 5 μmol/L [14C]-C18:1-CoA in the presence of 20 μmol/L LPC at 37°C for 30 min with 40 μg of clear lysate as described in method. CT775 was included as a control. After separation by thin-layer chromatography, [14C]-compounds were detected by phosphoimaging. [14C]-PC was used as a migration standard.
