Figure 5.
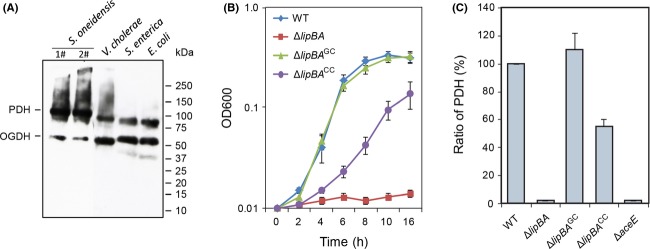
Physiological requirement of protein lipoylation in γ-proteobacteria. (A) Use of anti-LA Western blot to detect the requirement of protein lipoylation for γ-proteobacteria. Four species of γ-proteobacteria tested here include Escherichia coli, Salmonella enterica (S. enterica), Vibrio cholerae (V. cholerae) and Shewanella oneidensis (S. oneidensis). (B) Growth of the S. oneidensis lipBA mutant on lactate Complementation was carried out by either genetically (ΔlipBAGC, expressing a copy of the lipBA genes in trans) or chemically (ΔlipBACC, with the addition of lipoic acid of 3 pmol/mL). (C) Analyses for PDH activity The PDH dehydrogenase activities are given as micromoles of 3-acetylpyridine adenine dinucleotide reduced per milligram of protein per hour for extracts of the same number of cells estimated by OD600 readings. The relative activities (RA) were obtained by normalizing the values of other strains to the mean of wild-type values. In both (B) and (C), error bars represent standard deviations from at least three independent experiments. PDH, pyruvate dehydrogenase; OGDH, 2-oxoglutarate dehydrogenase; LA, Lipoic acid; kDa, kilo-dalton.
