Abstract
In diabetic nephropathy (DN), podocyte cytoskeletal rearrangement occurs followed by podocyte effacement and the development of proteinuria. PTEN (phosphatase and tensin homolog) is a ubiquitously expressed phosphatase that plays a critical role in cell proliferation, cytoskeletal rearrangement and motility. In mouse models of diabetes mellitus, PTEN expression is reportedly decreased in mesangial cells contributing to expansion of the mesangial matrix, but how PTEN in the podocyte influences the development of DN is unknown. We observed that PTEN expression is down-regulated in the podocytes of diabetic db/db mice and patients with DN. In cultured podocytes PTEN inhibition caused actin cytoskeletal rearrangement and this response was associated with unbalanced activation of small GTPases Rac1/Cdc42 and RhoA. In mice treated with PTEN inhibitor, actin cytoskeletal rearrangement occurred in podocytes and was accompanied by increased albumin excretion. We also created mice with an inducible deletion of PTEN selectively in podocytes. These mice exhibited increased albumin excretion and moderate foot process effacement. When the mice were challenged with a high fat diet, podocyte-specific knockout of PTEN resulted in substantially increased proteinuria and glomeruloclerosis compared to control mice fed a high fat diet or mice with PTEN deletion fed a normal diet. These results indicate that PTEN is involved in the regulation of cytoskeletal rearrangement in podocytes and that loss of PTEN predisposes to the development of proteinuria and DN.
Keywords: PTEN, Diabetic nephropathy, podocyte, cytoskeletal rearrangement
Introduction
Injuries to podocytes play a critical role in the development of many glomerular diseases including diabetic nephropathy (DN). An early event in the development of DN is a distinct cytoskeletal rearrangement [1;2] that drives a shortening and simplification of podocyte foot processes. It has been proposed that this process, known as effacement, may play an adaptive role, preventing podocyte detachment from the glomerular basement membrane [3]. These cytoskeletal rearrangements may also alter the integrity of slit diaphragms, resulting in albuminuria [4;5]. To date, the active reorganization of actin filaments in podocytes has been attributed to signal pathways that involve integrins, G protein– coupled receptors (GPCR), growth factor receptors, and Ca2+ influx pathways [6;7].
PTEN (phosphatase and tensin homolog) is a dual-function lipid and protein phosphatase that regulates a wide array of cellular processes including cell growth, migration and metabolism [8;9]. PTEN degrades phosphatidylinositol (3,4,5)-triphosphate (PIP3) into PIP2, and in this way opposes the actions of phosphatidylinositol-3 kinase (PI3K) [10]. There is evidence that PTEN regulates cytoskeletal dynamics. For example, down-regulation of PTEN increases fibroblast motility through stimulation of Rac1 and Cdc42 activity [11]. In podocytes, there is evidence that PIP3 induces actin polymerization by acting on signaling complexes mediated by nephrin and Neph1, in part through associated proteins such as PI3K [12]. Moreover, PTEN is down-regulated in mesangial cells in response to diabetes [13;14]. These observations raise the possibility that PTEN regulates cytoskeletal dynamics in podocytes, especially in response to stressful conditions occurring in the diabetic environment, such as hyperglycemia and hyperfiltration. To test this hypothesis, we examined PTEN expression in the podocytes of patients with DN and diabetic mice. In cultured podocytes we examined whether PTEN expression influences cytoskeletal remodeling and permeability to albumin. We assessed its role in determining the relative balance of RhoA, Rac1, and Cdc42 activation. Finally, we generated an inducible podocyte-specific PTEN knockout mouse and examined how this conditional PTEN deletion affects glomerular function and the progression of DN.
Materials and Methods
Cell culture and treatment podocyte culture, siRNA, Adenovirus-infection
Immortalized mouse podocytes (provided by Dr. Peter Mundel, Harvard University) have been described in detail previously [15]. Podocytes were maintained in RPMI-1640 medium (Life Technologies, Grand Island, NY) supplemented with 5% FBS, 2 mM L-glutamine, 100 mM sodium pyruvate, 100 U/ml penicillin, and 100 μg/ml streptomycin. To propagate podocytes, recombinant mouse γ-IFN (50 U/ml, R&D Systems, Minneapolis, MN) was added to culture medium with incubation at 33°C. To induce differentiation, podocytes were cultured at 37°C for 14 days prior to staining or treatment with Angiotensin II (2 nM) or TGF-β1 (10 ng/ml, R&D Systems). For polymerized actin (F-Actin) staining, differentiated podocytes were fixed with 4% paraformaldehyde for 5 min. After washing with PBS, the cells were stained using Alexa Fluor 488 phalloidin (Life Technologies) for 30 min. Quantitation of F-actin was carried out as described [16]. To knock down PTEN, differentiated podocytes were transfected with 6 μl of Smart Pool™ PTEN siRNA (Dharmacon, Waltham, MA) mixed with 100 μl siRNA transfection media (Life Technologies) for 30 min at room temperature. Subsequently, transfected podocytes were incubated 48 hr at 37°C. Scrambled siRNA served as the control. To overexpress PTEN, we infected podocytes with an adenovirus bearing human PTEN (Vector Biolab, Philadelphia, PA) for 36 hr. Control cells were infected with an adenovirus expressing β-galactosidase (Vector Biolab). PTEN protein level was confirmed by immunoblot. The podocyte permeability was measured as described previously with modifications [17;18].
Generation of iPPKO mice
All animal studies were approved by the BCM Institutional Animal Care and Use Committee. PTEN lox/lox mice (C57BL/6J background, Jackson lab, Bar Harbor, Maine) were crossed with mice in which inducible Cre recombinase (iCreERT2) was driven by the podocyte-specific podocin promoter [19]. At two months of age, PTEN lox/lox plus Cre positive mice and PTEN lox/lox only mice (served as Control) were injected with tamoxifen (2 mg/kg, i.p. 10 dosages) to induce PTEN knockout. After injection, iPPKO mice and their littermate controls were fed normal diet or challenged with a high fat diet (HFD) consisting of 23% protein, 35.8% fat, and 35.5% carbohydrate (Research Diets, New Brunswick, NJ) for 12 weeks as previously described [8]. Blood glucose was measured after 6 h fasting with the Accu-CHEK Advantage blood glucose meter (Accu-CHEK; Indianapolis, IN). Free fatty acids were measured in plasma samples using the NEFA™ kit (Biovision; Mountainview, CA).
Kidney tissue preparation
Before harvesting kidney tissues, mice were anesthetized and perfused with PBS via left ventricle puncture. Kidneys were then removed for further preparation. To visualize podocyte F-actin in glomeruli, we developed a frozen “tear-off” procedure designed to preserve polymerized actin filaments in mouse glomeruli. This method was developed because actin filaments can be disrupted during glomerular isolation procedures based on enzymatic digestion, including methods based on magnetic beads. Briefly, glass slides are pre-frozen in the chamber of a cryo-microtome for 5 min. Next, fresh whole mouse kidney is harvested, kidney capsule is removed, and exposed whole kidney is directly blotted onto the pre-frozen slides and then removed quickly, leaving only a thin layer of kidney tissue on the frozen slides. The slides were immersed in 4% paraformaldehyde for 5 min and then washed with PBS thoroughly before immunostaining. To prepare paraffin sections, kidney tissue was fixed in 4% paraformaldehyde and embedded in paraffin, cut to 4 μm thickness, stained with hematoxylin and eosin or PAS for morphological evaluation. Glomerular sclerosis score was performed as previously described [20], at least 30 glomeruli per mouse were evaluated. The mesangial matrix index was measured and calculated as the ratio of mesangial area to whole glomerular area (% area) [21;22] For isolation of whole glomeruli, mice were perfused with magnetic beads/HBSS (Life Technologies) via left ventricle puncture. Kidneys were removed and glomeruli were isolated as described [23]. For isolation of podocytes, glomeruli were harvested using Dynal™ beads and podocytes were detached by incubating glomeruli with trypsin-EDTA for 15 min at 37°C. After passing through a 40-μm cell strainer, podocytes were collected by centrifuging [24]. Preparation of tissue for electron microscopy was carried out with standard methods and sections were examined by transmission electron microscopy (Hitachi H-7500).
Immunofluorescence and confocal microscopy
For biopsy samples, previous clinical renal biopsy reports were reviewed to identify biopsies from normal kidney donors (3 cases) and those with the diagnosis of diabetic nephropathy (4 cases). Tissue sections from these biopsies were subjected to double immunofluorescent staining for PTEN and nephrin.
For the animal model, kidney sections or isolated glomeruli were incubated with primary antibodies overnight at 4°C, subsequently incubated with corresponding Alexa-fluor secondary antibodies (Life Technologies) for 30 min at room temperature. The following antibodies were used: anti-PTEN (Cell Signaling, Cambridge, MA), anti-WT1 (Santa Cruz Biotechnology, Santa Cruz, CA), anti-nephrin (R&D Systems). For F-actin staining, secondary antibody was replaced by Alexa Fluor 488 phalloidin. Nuclei were stained with DAPI. For morphometric analysis, samples were imaged using a Nikon A1-Rs inverted Laser Scanning microscope or a Nikon Eclipse I 80 fluorescence microscope using 40× or 100× objectives. To visualize podocyte F-actin in glomeruli, z-Stacks were collected at 0.12-μm intervals over a range of 1.0~1.5μm. The z-stacks were processed and recombined using image J software. 20 glomeruli were examined per sample slide in a random fashion.
Pull-down assay for small GTPases activities
RhoA, Rac1, and Cdc42 activities were determined by measuring Rhoketin or PAK1 pulled down by GTP-Rho and GTP-Rac/Cdc42, respectively. Pulled down GTPases were separated on 4–20% SDS-PAGE gel (Life Technologies) following the manufacturer's instructions (Cytoskeleton, Inc. Denver, CO). Loading controls were taken from 15% of total cell lysate from each sample in assays for RhoA, Rac1 and Cdc42.
24-hr albuminuria assessment
Urine was collected for 24 hr in metabolic cages and adjusted to same volume using pure water. Albumin standards were prepared by serial dilution of BSA (Sigma). Samples of standards or urine were mixed with 2XSDS-sample buffers (Sigma), boiled for 5 min, and analyzed by 10 % SDS-protein gel electrophoresis following Coomassie blue staining (Bio-RAD). The BSA bands (~65kD) were scanned and quantified using NIH imageJ.
Statistics
Results are presented as mean ± standard error of the mean (SEM). Data were analyzed using oneway ANOVA, P < 0.05 considered statistically significant.
Results
Down-regulation of PTEN in podocytes is present in diabetic nephropathy
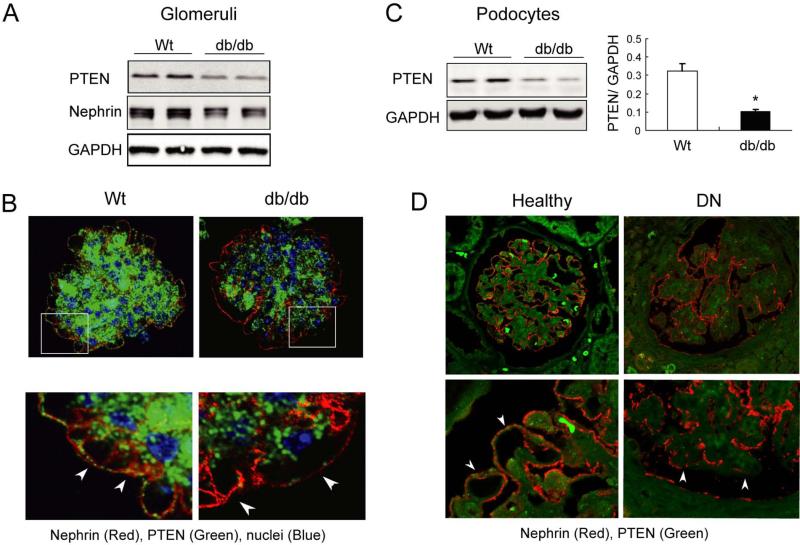
In initial experiments, we examined glomeruli isolated from six-month old db/db (C57BJ strain) and age-matched wild-type mice. Immunoblot analysis showed that PTEN expression was decreased in glomeruli of db/db mice compared to wild-type controls (Figure 1A). Nephrin expression was also decreased in glomeruli of db/db mice. Using double-immunostaining for PTEN and nephrin in glomeruli, we observed that, in wild-type mice, PTEN was localized in the mesangium and the outer layer of the glomerulus and co-localized with nephrin. But, in glomeruli from db/db mice, PTEN expression was markedly decreased in podocytes and the mesangium (Figure 1B). To confirm decreased PTEN in podocytes, we isolated podocytes from control and db/db mice. PTEN expression was markedly suppressed in the podocytes of db/db mice (Figure 1C).
Figure 1. PTEN is reduced in podocytes of mice and patients with diabetic nephropathy.
A: Immunoblots of PTEN and nephrin in isolated glomeruli from Wild-type (Wt) and db/db mice. B: confocal immunofluorescence microscopy of isolated glomeruli stained for PTEN and nephrin. PTEN expression is decreased in podocytes (nephrin positive cells, arrows) of db/db mice. C: immunoblot of PTEN in podocytes isolated from db/db mice. PTEN expression is significantly decreased. D: confocal immunofluorescence microscopy of glomeruli from healthy controls and patients with DN. PTEN (green signal) is decreased in podocytes (arrows) of patients with DN.
To evaluate the relevance of experimental results to DN in patients, we examined PTEN expression in the glomeruli of patients with DN and in healthy donor individuals. Double immunofluorescence staining revealed that, as in mice, PTEN signals were reduced in nephrin-positive cells as well as the mesangium in patients with DN compared to normal kidney (Figure 1D). This observation combined with the results from the mouse model suggest that PTEN is down-regulated in podocytes of patients with DN.
PTEN inhibition triggers cytoskeleton rearrangement in podocytes
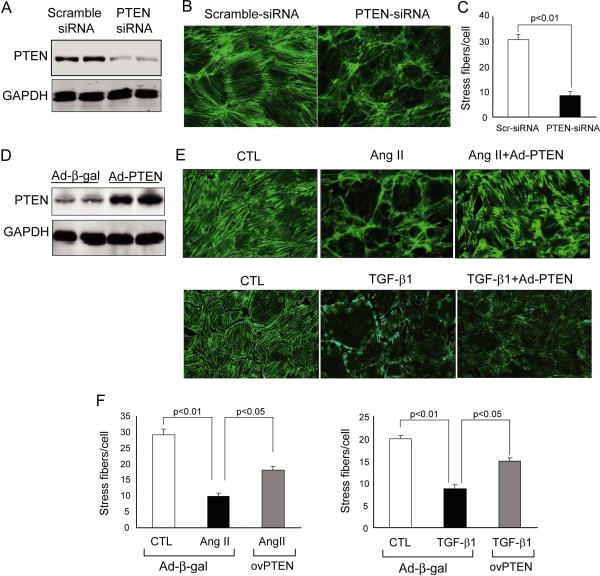
To determine if PTEN influences cytoskeletal remodeling in podocytes, we knocked down PTEN in differentiated mouse podocytes using siRNA interference. By western blotting analysis, we confirmed the knockdown efficiency of PTEN siRNA (Figure 2A). When the cells were treated with scrambled siRNA, the F-actin distribution in podocytes exhibited characteristic stress fibers that extended across the entire cell, so called “arch F-actin” [16;25]. After PTEN knockdown, the arch F-actin fibers were significantly decreased and there was a marked increase in disorganized, cortical F-actin (Figure 2B and C). A similar pattern was observed when PTEN activity was inhibited by bisperoxovanadium (BpV(HOpic), 100 ng/ml), a potent PTEN inhibitor [26;27]. BpV(HOpic) treatment reproduced the F-actin remodeling that was present in response to PTEN knockdown in podocytes (Supplemental Figure 1A). Knockdown of PTEN also results in a significant increase in the translocation of albumin across confluent podocyte monolayer vs. results in cells treated with scramble siRNA; similar results were observed when PTEN activity was inhibited by BpV(HOpic) (Supplemental Figure 1B). These results suggest that PTEN inhibition promotes cytoskeletal rearrangement and increase albumin permeability in podocyte cultures.
Figure 2. PTEN regulates cytoskeleton rearrangement in mouse podocytes cultures.
A: mouse podocytes were treated with PTEN siRNA or scramble-siRNA (as control) for 36h. Immunoblotting confirms the knockdown of PTEN. B: F-actin was visualized with Alexa Fluor 488-conjugated phalloidin. C: quantitation of Stress F-actin fibers (actin filament crossing entire cell). Stress fibers were counted from 50 cells per treatment and each treatment was repeated 3 times (SEM, n=3). D: Prior to Ang II or TGF-b1 treatment, podocytes were transfected with adenovirus expressing human PTEN or β-galactosidase (β-gal, as control) for 36 h. PTEN level was assessed by immunoblot. E: after 24 h of Ang II (2nM) or TGF-β1 (10ng/ml) treatment, F-actins were visualized with Alexa Fluor 488-conjugated phalloidin. F: Quantification of the stress fibers in E.
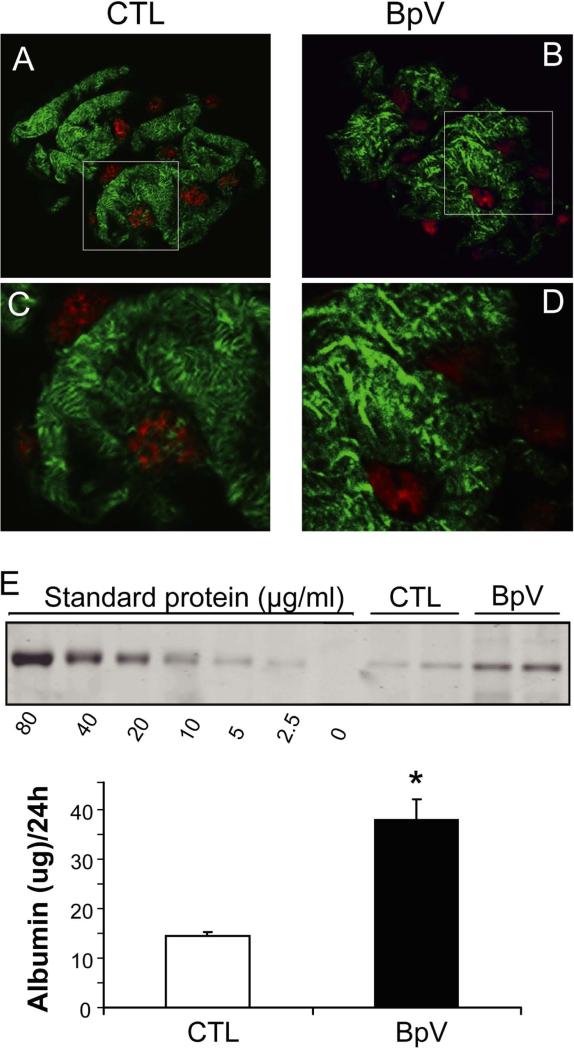
Angiotensin II (Ang II) and TGF-β1 can stimulate cytoskeletal rearrangement in podocytes, reportedly contributing to the development of DN [2;28]. To assess the relevance of PTEN in cytoskeletal rearrangement, we overexpressed PTEN in podocytes using an adenoviral vector (30 pfu/ml) for 36 h (Figure 2D). In control podocytes, exposure to Ang II or TGF-β1 caused a substantial cytoskeletal rearrangement and significant loss of arch F-actin fibers in podocytes. These responses were prevented in podocytes overexpressing PTEN (Figure 2E and F). Treatment with Ang II or TGF-β1 significantly increased the permeability for albumin in confluent podocyte monolayer and forced expression of PTEN largely prevented these responses (Supplemental Figure 1C). These results suggest that an increase in PTEN protects against cytoskeletal rearrangement and excessive albumin permeability in podocytes caused by detrimental stimuli. To extend these observations to the in vivo environment, we treated normal mice with BpV(HOpic) and investigated changes in podocyte F-actin using confocal microscopy. To preserve F-actin morphology in podocytes, we isolated glomeruli using a “frozen tear-off” procedure rather than methods based on enzymatic digestion (see Methods). In mice without treatment, the F-actin signals were located predominantly in primary and secondary foot processes of podocytes that cover the surface of the glomerular capillary, while F-actin was much less dense in the cell body of podocytes, where it typically exhibits a distinctly granular appearance (Figure 3A, C). Following BpV(HOpic) treatment (1mg/kg i.p.), there was a decrease in F-actin in the secondary processes with nucleation of F-actin in the primary processes (Figure 3B, D). These results indicate that PTEN inhibition stimulates remodeling of podocyte F-actin in vivo. To examine if this change in F-actin rearrangement affects glomerular function, we monitored 24 hr urinary albumin excretion after two BpV(HOpic) injections separated by 12 hr. As shown in Figure 3E, albumin excretion increased significantly with the inhibition of PTEN vs. results in control mice treated with vehicle. Collectively, these in vitro and in vivo observations support the hypothesis that PTEN inhibition stimulates podocyte cytoskeletal rearrangement leading to increased albumin excretion.
Figure 3. In mice, PTEN inhibition causes podocyte cytoskeletal rearrangement and albuminuria.
A and B: After C57B6 mice were injected with saline (CTL, A) or BpV (ip, 1mg/Kg, C) for 1h, preparation of glomeruli, and confocal immunoflorescence microscopy, were performed as described in Methods. F-fibers in podocytes were visualized with phalloidin (Green) and podocyte nuclei were labeled with WT1 (red). C and D: enlarged images from the areas indicated in the upper panels.
E: C57B6 mice were injected with BpV (ip, 1mg/Kg/12 h, two dosages) and 24h urines were collected. Urine samples were subjected to SDS electrophoresis and the amount of albumin determined as described in Methods.
PTEN regulates Rac1/Cdc42 and RhoA activities in podocytes
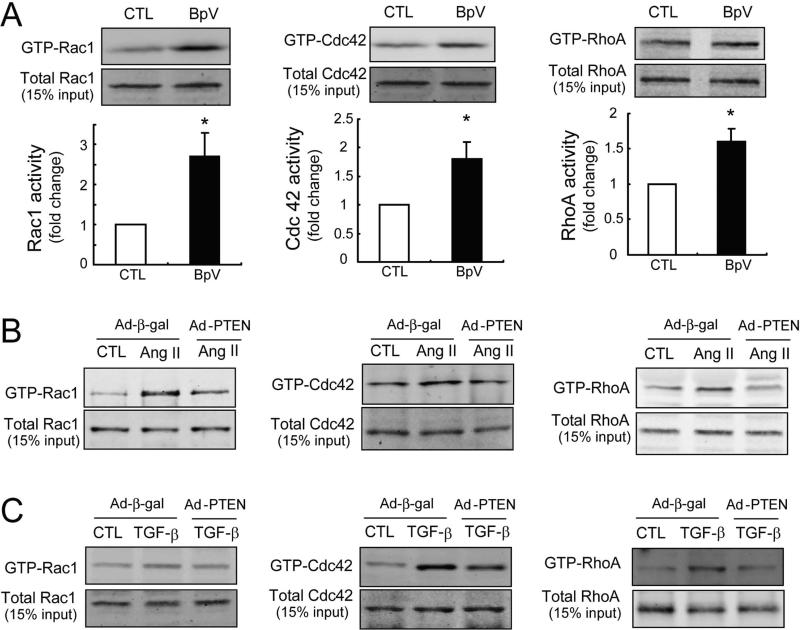
Small GTPases Rac1, Cdc42 and RhoA, have been proposed to regulate podocyte cytoskeletal rearrangement [29-31]. Activation of small GTPases requires GDP/GTP exchange factors (GEFs) [32;33]. GEFs in turn can be activated in a PI(3,4,5)P3-dependent manner but PTEN degrades PI(3,4,5)P3. We examined whether manipulating PTEN would change the activities of Rac1, Cdc42 or RhoA. We monitored the relative proportion of Rac1, Cdc42 or RhoA in a GTP-bound state, and inhibiting PTEN with BpV(HOpic) increased Rac1–GTP and Cdc42–GTP content in cultured podocytes by approximately 2.6-fold and 1.8-fold respectively. We also detected a 1.5-fold increase in RhoA activity (Figure 4A). Upon stimulation with Ang II, Rac1 was activated predominantly, accompanied by a slight increase in both Cdc42 and RhoA activities (Figure 4B). In response to TGF-β1, Cdc42 activity was much greater than that of Rac1 or RhoA (Figure 4C). PTEN overexpression blocked the activation of these small GTPases stimulated by Ang II or TGF-β1 (Figure 4B and C). Since PTEN can negatively regulate FAK phosphorylation, FAK could influence podocyte cytoskeletal remodeling [24]. However, there was no difference in tyrosine phosphorylation of FAK (p-FAK397) following BpV(HOpic) treatment, even though p-Akt was significantly increased by BpV(HOpic) (Supplemental Figure 2A). These results suggest that PTEN influences podocyte cytoskeletal rearrangement by regulating the activity of small GTPases.
Figure 4. PTEN regulates Rac1/Cdc42 and RhoA activities in mouse podocyte cultures.
A: The activities of small GTPases (Rac1, Cdc 42 and RhoA) in differentiated mouse podocytes were evaluated with or without BpV (100 ug/ml) treatment by Western blot after a pull-down assay (n=3 in each group, *<0.05).
B: Rac1, Cdc 42 and RhoA activities were evaluated by Western blot analysis after treatment with Ang II (ug/ml) for 24 h.
C: After exposure to TGF-β1 for 16 h, Rac1, Cdc 42 and RhoA activities were assessed by Western blotting. For the analysis of forced expression of PTEN, differentiated mouse podocytes were infected with adenoviruses: Ad-PTEN or Ad-β-gal (as control).
Podocyte-specific PTEN deletion is associated with increased albumin excretion
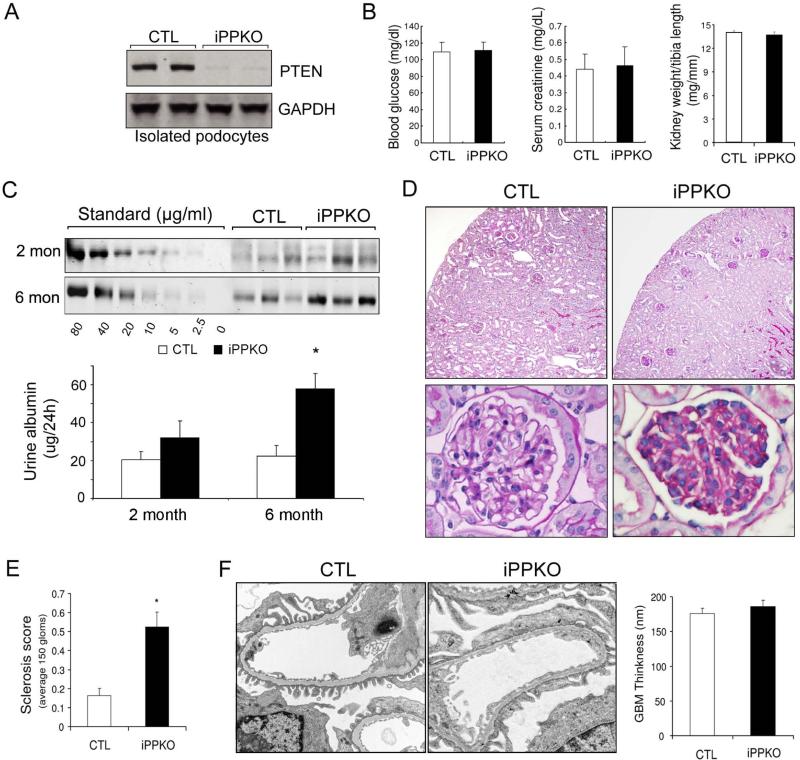
To further understand the impact of PTEN deletion on podocyte function in vivo, we generated mice with podocyte-specific PTEN knockout using loxP-flanked PTEN transgenic mice which were crossed with inducible, podocyte-specific Cre transgenic mice under the control of the podocin promoter[19;34]. The use of an inducible strategy allowed us to delete PTEN in the presence of tamoxifen in a temporal-specific manner. To selectively deplete PTEN from podocytes, the PTENlox/lox Cre+ (hereafter referred to as iPPKO) mice were administrated tamoxifen (1 mg/day/mouse, i.p.) for a total of 10 injections starting after 8 weeks of age. Tamoxifen treated PTEN lox/lox Cre− littermates mice served as controls. We examined PTEN expression by isolating podocytes from iPPKO and control mice 2 months after tamoxifen administration. Western blot revealed that PTEN expression was absent in podocytes isolated from iPPKO mice (Figure 5A). Using immunofluorescence double-staining, we also found that PTEN expression was not detectable in nephrin- positive cells or WT1-positive cells in the glomeruli of iPPKO mice (Supplemental Figure 2B). Podocyte-specific PTEN deletion did not affect kidney weight, blood glucose, or serum creatinine levels compared to control mice (Figure 5B). At 2 months after PTEN deletion, there was a trend towards an increase in urine albumin that did not reach statistical significance but. at 6 months, albumin excretion in iPPKO mice was moderately but significantly increased compared to values from littermate controls (Fig 5C). These results indicate that PTEN deletion in podocytes alters filtration function leading to albuminuria.
Figure 5. Podocyte-specific PTEN deletion (iPPKO) leads to albuminuria and glomerulosclerosis.
A: PTEN was absent in isolated podocytes of iPPKO mice vs. lox/lox (control) mice as assessed by Western blotting. B: Body weight, blood glucose and serum creatinine control were measured in control (CTL) and iPPKO mice (SEM, n=9). C: two or six months after PTEN deletion, 24h urines were collected and albumin levels were assessed using SDS electrophoresis (SEM, n=9~12, *<0.05).
D: 6 months after PTEN deletion, podocyte PTEN deletion was associated with mild to moderate glomerular sclerosis (Periodic acid-Schiff stain). E: Sclerosis index score (SEM, n=5, *<0.01). F: Electron micrographs from control and PTEN deleted glomeruli at 6 months of age show focal foot process effacement in iPPKO compared with controls. However, there is no GBM thickening in iPPKO mice (Scale bars represent 0.5 μm).
Loss of PTEN in podocytes results in moderate glomerulosclerosis
Histological examination of iPPKO mice kidneys revealed that approximately 16% of glomeruli displayed mild to moderate sclerosis at 2 months after PTEN deletion. At 6 months after PTEN deletion, however, the affected glomeruli were increased to ~50%. Although there was no collapse of the capillary tuft in glomeruli with sclerotic lesions, obliteration of glomerular capillaries was present (Figure 5D). At 6 months after PTEN deletion, the histological changes in glomeruli were also evaluated using a semi-quantitative sclerosis scoring method (Figure 5E). Since podocyte injuries are usually associated with slit diaphragm dysfunction [30;35;36], we examined nephrin expression in the glomeruli of iPPKO mice. By fluorescence microscopy, we observed a continuous, linear pattern of nephrin expression in the glomeruli of control mice. In contrast, the expression of nephrin in iPPKO mice displayed a more granular and diffuse pattern with reduced membrane nephrin (Supplemental Figure 2C). Electron microscopy demonstrated that podocytes in affected glomeruli exhibited moderate, focal foot process effacement; there was no systematic change in GBM thickness in affected glomeruli of iPPKO mice (Figure 5F). Taken together, these results indicate that loss of PTEN results in moderate podocyte effacement and glomerular abnormalities including sclerosis and obliteration of capillaries.
PTEN deletion in podocytes promotes the development of DN in mice fed a high fat diet
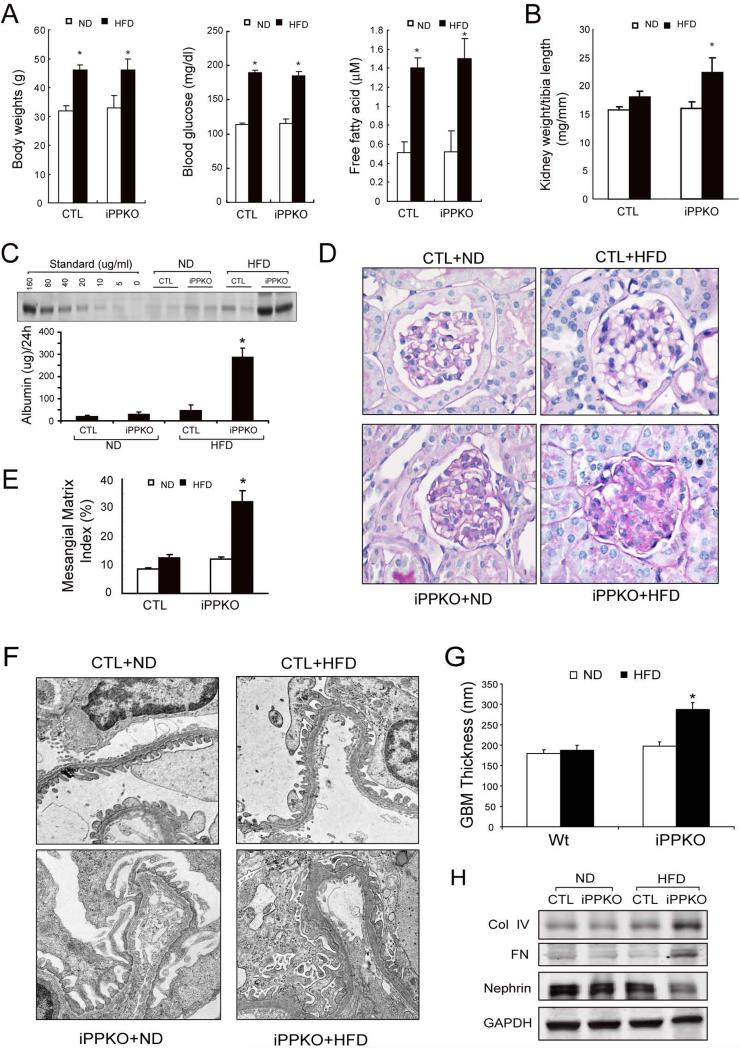
To investigate the role of PTEN deletion in the development of DN, we challenged iPPKO and control mice with a high fat diet (HFD) to determine if the absence of PTEN in podocytes accelerated the progression of DN. 2 month-old control and iPPKO mice were divided into 4 groups: Control; Control+HFD; iPPKO; and iPPKO+HFD, and maintained on HFD for 12 weeks. Although HFD significantly increased the body weights of control and iPPKO mice, there was no difference between Control+HFD and iPPKO+HFD groups. Blood glucose and plasma free fatty acids were also significantly higher in mice fed with HFD compared to the results of normal diet fed mice. However, there was no difference between Control+HFD and iPPKO+HFD mice (Figure 6A). But there was a marked increase in kidney weight (Figure 6B) and 24 h urinary albumin excretion in iPPKO+HFD mice vs. Control+HFD mice (Figure 6C). Consistent with the biochemical changes, periodic acid-Schiff (PAS) staining revealed glomerulosclerosis with expansion of mesangial matrix in the kidneys of iPPKO+HFD mice compared to results in control+HFD mice (Figure 6D). Electron microscopy demonstrated that there was severe foot process effacement and significant increase in GBM thickness in the glomeruli of iPPKO+HFD mice (Figure 6F and G). In addition, there was an increase in expression of collagen IV and fibronectin with a decrease in nephrin expression in isolated glomeruli of HFD-fed, iPPKO mice (Figure 6H). Taken together, these results suggest that loss of PTEN in podocytes promotes the progression of DN.
Figure 6. Deletion of PTEN in podocytes accelerates the development of DN and proteinuria in mice fed a high fat diet.
A: iPPKO and control (CTL) mice were fed with a normal diet (ND) or a high fat diet (HFD) for 12 weeks. Body weight, blood glucose and free fatty acid were measured in each group (SEM, n=9, *p<0.01).
B: Kidney weight/tibia length ratio in each group. C: 24 h albumin excretion of control and iPPKO mice fed with ND or HFD was assessed using SDS electrophoresis (n=9, *p<0.01). D: Periodic acid–Schiff staining of glomeruli from control and iPPKO mice fed with ND or HFD. E: Quantification of mesangial matrix index in control and iPPKO mice fed with ND or HFD; 25 glomeruli/mouse were examined (n=9 in each group, *p<0.05). F: Representative electron microscopy images of glomerular basement membrane (GBM) thickening. G: quantification of GBM thickness in each group. H: Representative Western blot of isolated glomeruli lysates from control and iPPKO mice fed with ND or HFD. GAPDH was used as loading control.
Discussion
In the present study, we have observed a decrease in PTEN levels in podocytes of db/db mice as well as in patients with DN. We also observed that podocyte-specific PTEN deletion in mice caused podocyte cytoskeletal rearrangements that were accompanied by albuminuria. PTEN inhibition and deletion altered cytoskeletal dynamics, resulting in changes in the permeability and barrier function of podocytes. Finally, podocyte PTEN deletion promoted the development of albuminuria and glomerulopathy in mice maintained on a HFD.
It was recently shown that deletion of insulin receptors from podocytes decreased IRS1-associated PI3K activity which was followed by podocyte apoptosis and glomerulosclerosis [37]. Since PTEN can oppose PI3K activity owing to its effects on PIP3, we initially hypothesized that deletion of PTEN would enhance insulin signaling and would thereby produce protective effects on glomerular function. Instead we observed that a loss of PTEN in podocytes resulted in markedly more severe glomerular lesions in mice fed a HFD. The likely explanation is that insulin receptors are primarily localized in the cell body of podocytes, whereas PTEN is localized predominantly in the foot processes. Therefore signaling through this system may be spatially isolated and they are therefore likely to play distinct and independent roles in podocyte function. Thus, PTEN signaling may primarily regulate the actin dynamics of foot processes, whereas insulin signaling may primarily occur in the podocyte cell body to reduce apoptosis [37;38]. There is considerable precedent in other cell types that the function of PTEN depends on its subcellular localization [39-41], and one would expect that signaling can be especially compartmentalized in highly polarized cells such as podocytes.
In cultured podocytes we observed that PTEN inhibition resulted in marked rearrangements of F-actin stress fibers, which was accompanied by an increase in the permeability of confluent monolayer cultures. Conversely, when PTEN was over-expressed in podocytes, Ang II− or TGF-β−induced hyperpermeability was blocked. The mechanisms whereby PTEN can alter cytoskeletal dynamics are likely to be complex. However we observed that PTEN inhibition increased RhoA/Rac1/Cdc42 signaling, and it is possible that those effects arise from direct actions of PTEN on PIP3 [42]. A substantial body of evidence implicates these small G proteins in the regulation of podocyte foot process [3;7], and these effects could partially explain increased susceptibility to DN, possibly by promoting effacement or detachment of podocytes from the basement membrane.
The iPPKO mouse allowed us to study the effects of PTEN deletion selectively in podocytes. There are several advantages to the inducible knockout strategy that we used in this study: first, most patients develop DN during adulthood and the inducible knockout model allows us to mimic this pattern. The glomeruli of mice continue to develop throughout the first few months of their lives [43], so an inducible knockout system circumvents potentially confounding developmental defects that could occur with PTEN deletion. We observed that there was no significant albuminuria two months after deletion of PTEN only in podocytes. This result is different from the response measured after PTEN inhibition with BpV(Hopic); where there was a ~2.2 fold increase in albumin excretion (Figure 3E). A potential interpretation is that the PTEN inhibitor, BpV(Hopic), may suppress other phosphatases [44], or other enzymes in podocytes which may increase protein leakage across the filtration barrier [45]. It is also possible that administration of PTEN inhibitors increased the permeability of the glomerular endothelial cells or impaired albumin re-absorption in proximal tubular cells, thus contributing to the proteinuria detected in these mice. In addition, during a gradual genetic knockdown, there is ample time for cells to at least partially compensate by increasing other phosphatases activities, whereas, by comparison, the effects of inhibitors are quick and abrupt, which does not allow for compensation [34].
Although these results provided evidence that PTEN deletion causes podocyte dysfunction, the absence of PTEN in podocytes did not mirror all of the histopathological features of DN in patients, as there was no change in GBM thickness, mesangial expansion, glomerular or arteriolar hyalinosis, or Kimmelstiel-Wilson nodules. Moreover, inflammation and fibrosis were minimal in the glomerular lesions. These results indicate that loss of PTEN alone is not sufficient to cause diabetic nephropathy under normal conditions. Since down-regulation of PTEN was present before there was discernable mesangial expansion, it is possible that a decrease in PTEN contributes to microalbuminuria and early changes of diabetic glomerulopathy even before pathological changes are visible by light microscopy.
There is now evidence for “cross-talk” between podocytes and glomerular endothelial cells [46]. We found that at six months after podocyte-specific PTEN deletion, there was swelling of glomerular endothelial cells, followed by obliteration of some of the glomerular capillaries, suggesting that PTEN deletion in podocytes interrupts communication between podocytes and glomerular endothelial cells. We recognized that this association must be explored further to examine in more detail the role of PTEN in signaling between podocytes and glomerular endothelial cells.
In summary, our results from patients with DN, from cultured podocytes, and from an inducible knockout mouse model demonstrate that podocyte PTEN plays an important role in regulating actin cytoskeletal dynamics, which are critical for maintenance of glomerular filtration. Down-regulation of PTEN in podocytes gives rise to a dysregulation in actin dynamics that favours foot process effacement and albuminuria, facilitating the development of diabetic nephropathy.
Supplementary Material
Supplemental Figure 1: A: Mouse podocyte cultures were treated with the PTEN inhibitor BpV(Hopic) for 1 h. Stress fibers (Factin) were labeled with Alexa Fluor 488-conjugated phalloidin (Green).
B: Podocyte permeability was determined by measuring the amount of albumin crossing a podocyte monolayer during the first hour after albumin (40 mg/ml) was added (SEM, n=7). Left panel: podocyte permeability 36 h after siRNA treatment. Right panel: permeability was measured after BpV (100 ug/ml) was added simultaneously with albumin.
C: Podocytes were transfected with adenovirus expressing human PTEN (Ad-PTEN) or β-galactosidase (in CTL and Ang II or TGF-β1 group) for 36 h. Permeability was then determined after 24 h of Ang II incubation (left panel) or TGF-β1 treatment (right panel) (SEM, n=9).
Supplemental figure 2 A: The phosphorylation of FAK (Y 392) was evaluated by Western blotting with or without BpV (100 μg/ml) treatment (left panel). PTEN inhibition does not increase the phosphorylation of FAK. However, the phosphorylation of Akt (ser 473) was increased by BpV treatment (right panel).
B: Immunofluoresent staining of kidney sections. PTEN (green) is absent in nephrin (left panel, red) or WT1 (right panel, red) positive cells of iPPKO mice but is present in these cells of lox/lox (CTL) mice. Nuclei are stained with DAPI (Blue). The enlarged images (lower panel) are the areas indicated in the upper panel.
C: Immunofluorescent staining of nephrin (red) reveals a granular and diffuse pattern with reduced expression in glomeruli of iPPKO mice.
Acknowledgments
We thank Dr William E. Mitch for helpful comments and encouragement. This work was supported byNSFC81270803 and Shanghai Pujiang program Foundation (11PJ1412400 to J.X), and by National Institutes of Health (USA) grants (5RO1 AR063686 and P30-DK079638 to Z.H). Hui Peng is supported by NSFC81170678. Stuart Dryer is supported by a contract from Pfizer Inc. and by a grant from the Juvenile Diabetes Research Foundation.
Footnotes
Author contributions
J.L, Y.S, H.P, X.S, Z.H and J.X carried out experiments, study design and data analysis; Y.W, L.T, S.D, Z.H and J.X interpreted the data; and S.T, S.D, Z.H and J.X were involved in writing the paper. All authors had final approval of the submitted versions.
The authors declare that there are no conflicts of interest.
Reference List
- 1.Faul C, Asanuma K, Yanagida-Asanuma E, et al. Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton Trends. Cell Biol. 2007;17:428–437. doi: 10.1016/j.tcb.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Herman-Edelstein M, Thomas MC, Thallas-Bonke V, et al. Dedifferentiation of immortalized human podocytes in response to transforming growth factor-beta: a model for diabetic podocytopathy. Diabetes. 2011;60:1779–1788. doi: 10.2337/db10-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kriz W, Shirato I, Nagata M, et al. The podocyte's response to stress: the enigma of foot process effacement. Am J Physiol Renal Physiol. 2013;304:F333–F347. doi: 10.1152/ajprenal.00478.2012. [DOI] [PubMed] [Google Scholar]
- 4.Haraldsson B, Nystrom J, Deen WM. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev. 2008;88:451–487. doi: 10.1152/physrev.00055.2006. [DOI] [PubMed] [Google Scholar]
- 5.Patrakka J, Tryggvason K. New insights into the role of podocytes in proteinuria. Nat Rev Nephrol. 2009;5:463–468. doi: 10.1038/nrneph.2009.108. [DOI] [PubMed] [Google Scholar]
- 6.Dryer SE, Reiser J. TRPC6 channels and their binding partners in podocytes: role in glomerular filtration and pathophysiology. Am J Physiol Renal Physiol. 2010;299:F689–F701. doi: 10.1152/ajprenal.00298.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greka A, Mundel P. Cell biology and pathology of podocytes. Annu Rev Physiol. 2012;74:299–323. doi: 10.1146/annurev-physiol-020911-153238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hu Z, Wang H, Lee IH, et al. PTEN inhibition improves muscle regeneration in mice fed a high-fat diet. Diabetes. 2010;59:1312–1320. doi: 10.2337/db09-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Z, Dong X, Wang Z, et al. Regulation of PTEN by Rho small GTPases. Nat Cell Biol. 2005;7:399–404. doi: 10.1038/ncb1236. [DOI] [PubMed] [Google Scholar]
- 10.Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 11.Liliental J, Moon SY, Lesche R, et al. Genetic deletion of the Pten tumor suppressor gene promotes cell motility by activation of Rac1 and Cdc42 GTPases. Curr Biol. 2000;10:401–404. doi: 10.1016/s0960-9822(00)00417-6. [DOI] [PubMed] [Google Scholar]
- 12.Zhu J, Sun N, Aoudjit L, et al. Nephrin mediates actin reorganization via phosphoinositide 3-kinase in podocytes. Kidney Int. 2008;73:556–566. doi: 10.1038/sj.ki.5002691. [DOI] [PubMed] [Google Scholar]
- 13.Kato M, Putta S, Wang M, et al. TGF-beta activates Akt kinase through a microRNA-dependent amplifying circuit targeting PTEN. Nat Cell Biol. 2009;11:881–889. doi: 10.1038/ncb1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahimainathan L, Das F, Venkatesan B, Choudhury GG. Mesangial cell hypertrophy by high glucose is mediated by downregulation of the tumor suppressor PTEN. Diabetes. 2006;55:2115–2125. doi: 10.2337/db05-1326. [DOI] [PubMed] [Google Scholar]
- 15.Mundel P, Reiser J, Zúñiga Mejía Borja A, Pavenstädt H, Davidson GR, Kriz W, Zeller R. Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res. 1997 Oct 10;236(1):248–58. doi: 10.1006/excr.1997.3739. [DOI] [PubMed] [Google Scholar]
- 16.Tian D, Jacobo SM, Billing D, et al. Antagonistic regulation of actin dynamics and cell motility by TRPC5 and TRPC6 channels. Sci Signal. 2010;3:ra77. doi: 10.1126/scisignal.2001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma K, Ramachandrarao S, Qiu G, et al. Adiponectin regulates albuminuria and podocyte function in mice. J Clin Invest. 2008;118:1645–1656. doi: 10.1172/JCI32691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maruo N, Morita I, Shirao M, Murota S. IL-6 increases endothelial permeability in vitro. Endocrinology. 1992;131:710–714. doi: 10.1210/endo.131.2.1639018. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Wang Y, Long J, et al. Tamoxifen-inducible podocyte-specific iCre recombinase transgenic mouse provides a simple approach for modulation of podocytes in vivo. Genesis. 2010;48:446–451. doi: 10.1002/dvg.20635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ma LJ, Fogo AB. Model of robust induction of glomerulosclerosis in mice: importance of genetic background. Kidney Int. 2003;64:350–355. doi: 10.1046/j.1523-1755.2003.00058.x. [DOI] [PubMed] [Google Scholar]
- 21.Kolavennu V, Zeng L, Peng H, et al. Targeting of RhoA/ROCK signaling ameliorates progression of diabetic nephropathy independent of glucose control. Diabetes. 2008;57:714–723. doi: 10.2337/db07-1241. [DOI] [PubMed] [Google Scholar]
- 22.Long J, Wang Y, Wang W, et al. MicroRNA-29c is a signature microRNA under high glucose conditions that targets Sprouty homolog 1, and its in vivo knockdown prevents progression of diabetic nephropathy. J Biol Chem. 2011;286:11837–11848. doi: 10.1074/jbc.M110.194969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takemoto M, Asker N, Gerhardt H, et al. A new method for large scale isolation of kidney glomeruli from mice Am. J Pathol. 2002;161:799–805. doi: 10.1016/S0002-9440(10)64239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma H, Togawa A, Soda K, et al. Inhibition of podocyte FAK protects against proteinuria and foot process effacement. J Am Soc Nephrol. 2010;21:1145–1156. doi: 10.1681/ASN.2009090991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asanuma K, Yanagida-Asanuma E, Faul C, et al. Synaptopodin orchestrates actin organization and cell motility via regulation of RhoA signalling. Nat Cell Biol. 2006;8:485–491. doi: 10.1038/ncb1400. [DOI] [PubMed] [Google Scholar]
- 26.Walker CL, Walker MJ, Liu NK, et al. Systemic bisperoxovanadium activates Akt/mTOR, reduces autophagy, and enhances recovery following cervical spinal cord injury. PLoS One. 2012;7:e30012. doi: 10.1371/journal.pone.0030012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Castaldi L, Serra C, Moretti F, et al. Bisperoxovanadium, a phospho-tyrosine phosphatase inhibitor, reprograms myogenic cells to acquire a pluripotent, circulating phenotype. FASEB J. 2007;21:3573–3583. doi: 10.1096/fj.06-7454com. [DOI] [PubMed] [Google Scholar]
- 28.Remuzzi G, Schieppati A, Ruggenenti P. Clinical practice. Nephropathy in patients with type 2 diabetes. N Engl J Med. 2002;346:1145–1151. doi: 10.1056/NEJMcp011773. [DOI] [PubMed] [Google Scholar]
- 29.Faul C, Donnelly M, Merscher-Gomez S, et al. The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine. A Nat Med. 2008;14:931–938. doi: 10.1038/nm.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mundel P, Reiser J. Proteinuria: an enzymatic disease of the podocyte? Kidney Int. 2010;77:571–580. doi: 10.1038/ki.2009.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei C, Moller CC, Altintas MM, et al. Modification of kidney barrier function by the urokinase receptor. Nat Med. 2008;14:55–63. doi: 10.1038/nm1696. [DOI] [PubMed] [Google Scholar]
- 32.van AL, Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- 33.Han J, Luby-Phelps K, Das B, et al. Role of substrates and products of PI 3-kinase in regulating activation of Rac-related guanosine triphosphatases by Vav. Science. 1998;279:558–560. doi: 10.1126/science.279.5350.558. [DOI] [PubMed] [Google Scholar]
- 34.Wang W, Wang Y, Long J, et al. Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells. Cell Metab. 2012;15:186–200. doi: 10.1016/j.cmet.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kriz W, Endlich K. Podocytes and disease: introduction. Semin Nephrol. 2012;32:305–306. doi: 10.1016/j.semnephrol.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 36.Johnstone DB, Holzman LB. Clinical impact of research on the podocyte slit diaphragm. Nat Clin Pract Nephrol. 2006;2:271–282. doi: 10.1038/ncpneph0180. [DOI] [PubMed] [Google Scholar]
- 37.Welsh GI, Hale LJ, Eremina V, et al. Insulin signaling to the glomerular podocyte is critical for normal kidney function. Cell Metab. 2010;12:329–340. doi: 10.1016/j.cmet.2010.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tejada T, Catanuto P, Ijaz A, et al. Failure to phosphorylate AKT in podocytes from mice with early diabetic nephropathy promotes cell death. Kidney Int. 2008;73:1385–1393. doi: 10.1038/ki.2008.109. [DOI] [PubMed] [Google Scholar]
- 39.Funamoto S, Meili R, Lee S, et al. Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell. 2002;109:611–623. doi: 10.1016/s0092-8674(02)00755-9. [DOI] [PubMed] [Google Scholar]
- 40.Martin-Belmonte F, Gassama A, Datta A, et al. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell. 2007;128:383–397. doi: 10.1016/j.cell.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gil A, ndres-Pons A, Pulido R. Nuclear PTEN: a tale of many tails. Cell Death Differ. 2007;14:395–399. doi: 10.1038/sj.cdd.4402073. [DOI] [PubMed] [Google Scholar]
- 42.Randazzo PA, Nie Z, Miura K, Hsu VW. Molecular aspects of the cellular activities of ADP- ribosylation factors. Sci STKE. 2000;2000:re1. doi: 10.1126/stke.2000.59.re1. [DOI] [PubMed] [Google Scholar]
- 43.Sivaskandarajah GA, Jeansson M, Maezawa Y, et al. Vegfa protects the glomerular microvasculature in diabetes. Diabetes. 2012;61:2958–2966. doi: 10.2337/db11-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Spinelli L, Lindsay YE, Leslie NR. PTEN inhibitors: An evaluation of current compounds. Adv Biol Regul. 2014 doi: 10.1016/j.jbior.2014.09.012. [DOI] [PubMed] [Google Scholar]
- 45.Reiser J, Pixley FJ, Hug A, et al. Regulation of mouse podocyte process dynamics by protein tyrosine phosphatases rapid communication. Kidney Int. 2000;57:2035–2042. doi: 10.1046/j.1523-1755.2000.00070.x. [DOI] [PubMed] [Google Scholar]
- 46.Kim SI, Lee SY, Wang Z, et al. TGF-beta-Activated Kinase 1 Is Crucial in Podocyte Differentiation and Glomerular Capillary Formation. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2013030252. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: A: Mouse podocyte cultures were treated with the PTEN inhibitor BpV(Hopic) for 1 h. Stress fibers (Factin) were labeled with Alexa Fluor 488-conjugated phalloidin (Green).
B: Podocyte permeability was determined by measuring the amount of albumin crossing a podocyte monolayer during the first hour after albumin (40 mg/ml) was added (SEM, n=7). Left panel: podocyte permeability 36 h after siRNA treatment. Right panel: permeability was measured after BpV (100 ug/ml) was added simultaneously with albumin.
C: Podocytes were transfected with adenovirus expressing human PTEN (Ad-PTEN) or β-galactosidase (in CTL and Ang II or TGF-β1 group) for 36 h. Permeability was then determined after 24 h of Ang II incubation (left panel) or TGF-β1 treatment (right panel) (SEM, n=9).
Supplemental figure 2 A: The phosphorylation of FAK (Y 392) was evaluated by Western blotting with or without BpV (100 μg/ml) treatment (left panel). PTEN inhibition does not increase the phosphorylation of FAK. However, the phosphorylation of Akt (ser 473) was increased by BpV treatment (right panel).
B: Immunofluoresent staining of kidney sections. PTEN (green) is absent in nephrin (left panel, red) or WT1 (right panel, red) positive cells of iPPKO mice but is present in these cells of lox/lox (CTL) mice. Nuclei are stained with DAPI (Blue). The enlarged images (lower panel) are the areas indicated in the upper panel.
C: Immunofluorescent staining of nephrin (red) reveals a granular and diffuse pattern with reduced expression in glomeruli of iPPKO mice.