Table 3.
MPC4 and CC50 values of analogs with variations in R5 and R6 groups
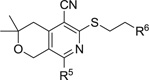 | ||||
|---|---|---|---|---|
| Compound | R6 | MPC4 (µM) | CC50 (µM) |
|
| LVX | PIP | |||
| R5 = morpholinyl | ||||
| 20a | cyano | ≥100 | ≥100 | >100 |
| 20b | tetrazol-5-yl | ≥100 | ≥100 | 30.2 |
| 20c | pyridin-2-yl | 50 | 25 | 78.1 |
| 20d | pyridin-3-yl | 25 | 3.1 | >100 |
| 20e | 2-methoxyphenyl | 3.1 | 3.1 | >100 |
| 20f | 3-methoxyphenyl | 2.4 | 1.6 | >100 |
| 20g | 4-methoxyphenyl | 6.3 | 3.1 | >100 |
| 20h | 3-acetoxyphenyl | 1.6 | 1.6 | 48.3 |
| 20i | 2-fluorophenyl | ≥100 | ≤1.6 | >100 |
| 20j | 3-fluorophenyl | ≥100 | 4.7 | >100 |
| 20k | 4-fluorophenyl | 6.3 | 3.1 | 59.0 |
| 20l | 3-chlorophenyl | 3.1 | 3.1 | >100 |
| 20m | 3-indolyl | 12.5 | 2.4 | 44.8 |
| 20n | 3-benzofuranyl | >100 | 6.3 | >100 |
| 20o | 3-cyanophenyl | 12.5 | 3.9 | >100 |
| 20p | 3- (tetrazol-5-yl)phenyl | ≥100 | 12.5 | 20.7 |
| 20q | 4-(SO3H)phenyl | ≥100 | ≥100 | 17.2 |
| 20r | 3,4-dimethoxyphenyl | 1.3 | 0.8 | 69.4 |
| R5 = 4-(2-methoxyethyl)-piperazinyl | ||||
| 21a | pyridin-2-yl | 12.5 | 3.1 | >100 |
| 21b | pyridin-3-yl | ≥100 | 25 | >100 |
| 21c | pyridine-4-yl | 6.3 | 50 | 95.9 |
| 21d | 1,3-dioxan-2-yl | ≥100 | 25 | >100 |
| 21e | 3-methoxyphenyl | 6.3 | 4.7 | 46.5 |
| 21f | 4-methoxyphenyl | 6.3 | 3.1 | 34.8 |
| 21g | 3,4-dimethoxyphenyl | 1.6 | 0.4 | 38.8 |
| 21h | 2-fluorophenyl | 12.5 | 9.4 | 30.6 |
| 21i | 3-fluorophenyl | ≥100 | 6.3 | 37.9 |
| 21j | 3-bromophenyl | 12.5 | 2.8 | 75.1 |
| 21k | phenoxy | ≥100 | 12.5 | 33.9 |
| R5 = 2,6-dimethyl-morpholinyl | ||||
| 22a | pyridin-3-yl | 12.5 | 3.1 | 42.6 |
| 22b | 3-methoxyphenyl | 9.4 | 1.6 | >100 |
| 22c | 3-fluorophenyl | 1.6 | 1.6 | >100 |
| 22d | 4-BocNHphenyl | 0.8 | 0.4 | >100 |
| 22e | 4-aminophenyl | 0.8 | 0.8 | 52.2 |
| 22f | 4-(CH3CONH)phenyl | 0.1 | 0.1 | 60.5 |
| 22g | 4-(CF3CONH)phenyl | 1.6 | 1.6 | 64.0 |
| 22h | 4-(CH3SO2NH)phenyl | 6.3 | 6.3 | 67.0 |
| 22i | 4-acrylamidophenyl | 0.1 | 0.05 | 62.4 |
| 22j | 4-[(CH3)3CONH]phenyl | ≥100 | ≥100 | >100 |
| 22k | 4-guanidinophenyl | 0.8 | 0.8 | 12.0 |
