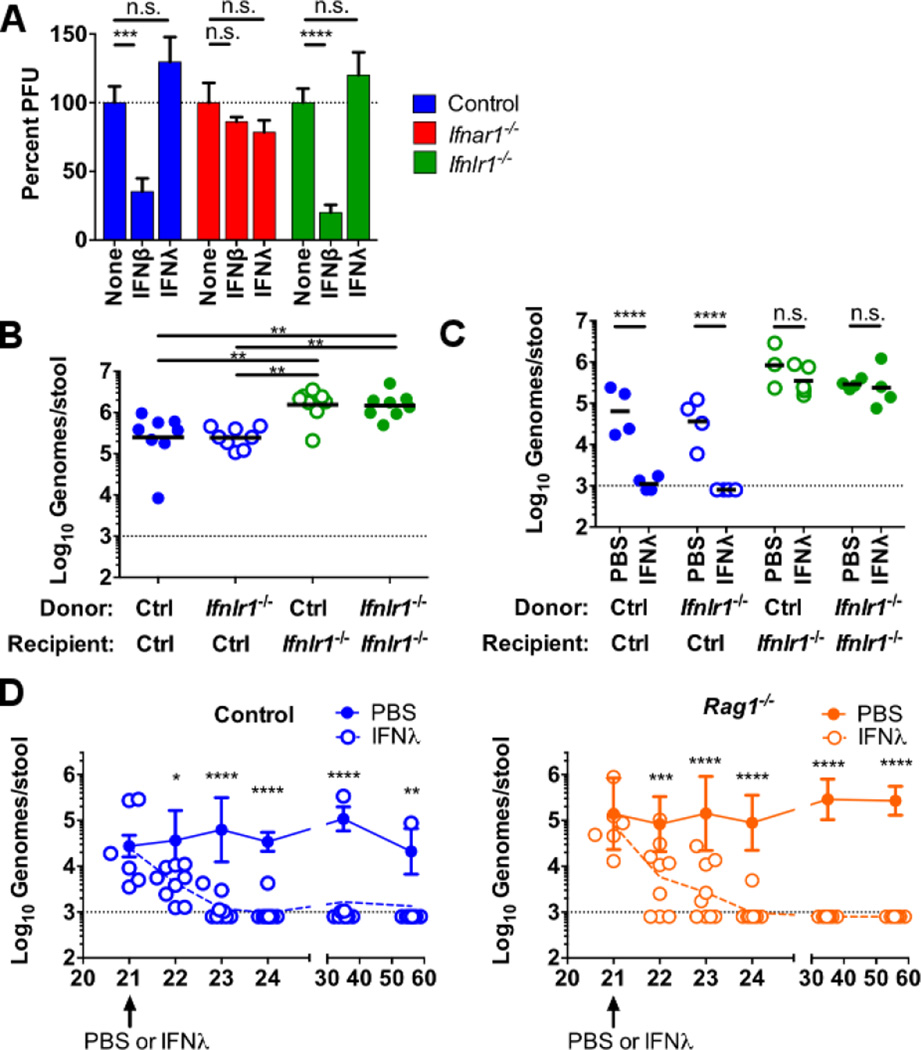
Figure 4. IFN-λ durably clears enteric MNoV persistence through effects on radiation-insensitive cells in the absence of adaptive immunity.
(A) BMDCs were treated with media, 100 IU/mL IFN-β, or 100 ng/mL IFN-λ for 24 hours, then inoculated with CR6 at a multiplicity of infection of five, and viral titers were determined by plaque assay 12 hours later. Data are pooled from three independent experiments performed in triplicate and normalized to untreated. (B and C) Bone marrow chimeras were generated using control and Ifnlr1−/− donor and recipient mice as indicated and 8 to 10 weeks later were orally inoculated with 106 PFUs of CR6. (B) MNoV shedding in feces was quantified on day 14. (C) A single 25-µg dose of IFN-λ or PBS was administered intraperitoneally 21 days after inoculation and MNoV shedding was quantified on day 23. Data in (B) and (C) are pooled from two independent experiments and shown as individual mice. (D) Control or Rag1−/− mice orally inoculated with CR6 were treated 21 days later with a single 25-µg dose of IFN-λ. Shedding was monitored for 35 days postinjection. Dashed lines in (B) to (D) represent limit of detection. Statistical significance was determined by one-way (B) or two-way (A, C, D) ANOVA. n.s., not significant (P > 0.05); *P ≤ 0.05, **P ≤ 0.01, *** P ≤ 0.001, ****P ≤ 0.0001. Error bars in (A) denote SEM and in (D) denote SD.