Abstract
Background and Objective
Uveal melanoma (UM) is the most common primary intraocular cancer, however the molecular features that predict response to therapy are poorly understood. Our objective was to determine whether gene expression profiling (GEP) is associated with rate of tumor regression following I-125 plaque brachytherapy for UM.
Methods
Retrospective review of 138 patients with posterior UM treated with I-125 plaque brachytherapy in which GEP class and 3-month post-radiation ultrasonographic tumor thickness were available. Statistical analysis was performed using T-test and Fisher’s exact test.
Results
GEP class assignment was class 1 in 83 (60.1%) and class 2 in 55 (39.9%) patients. Mean patient age was 60.9 years for class 1 and 68.1 years for class 2 tumors (P=0.002). Mean initial tumor diameter was 13.0 mm for class 1 and 14.1 mm for class 2 tumors (P=0.02). Mean initial tumor thickness was 5.2 mm for class 1 and 6.1 mm for class 2 tumors (P=0.047). Mean reduction in tumor thickness at 3-month post-radiation 26.5% for class 1 and 16.7% for class 2 tumors (P=0.03) after I-125 plaque radiotherapy.
Conclusion
Class 1 tumors exhibit more rapid early tumor regression than class 2 tumors following I-125 plaque radiotherapy.
INTRODUCTION
Uveal melanoma (UM) is the most common primary intraocular cancer and is most commonly treated in the U.S. with Iodine-125 episcleral plaque brachytherapy (EPB).1 Although EPB is associated with excellent local tumor control, 50% of patients will go on to develop metastasis. Gene expression profiling (GEP) accurately classifies patients according to metastatic risk: class 1 tumors have a low risk and class 2 tumors have a high risk of metastasis independent of primary tumor treatment.2 A GEP clinical test that is now commercially available is the most accurate molecular prognostic test available for uveal melanoma and the only one to be validated in a prospective, multi-center study.3 The objective of this study was to determine whether GEP class status predicts tumor regression at 3 months post-EPB.
PATIENTS/MATERIALS AND METHODS
To determine whether GEP correlates with response to Iodine-125 plaque brachytherapy, we studied 138 patients who (1) underwent I-125 plaque brachytherapy for posterior uveal melanoma, (2) obtained the GEP test from a fine needle biopsy, and (3) had ultrasonographic tumor thickness measurement at baseline and at the 3 month post-operative visit by the same examiner (J.W.H.). These 138 patients were treated between 6/1/01 – 10/28/11, and were identified from a total of 563 patients with choroidal melanoma treated at Washington University over a 15-year period, from 11/1/96 – 10/28/11. The latest date of follow-up was 7/19/14. The study was approved by the Washington University Institution Review Board.
RESULTS
Study patients included 67 (48.5%) women and 71 (51.5%) men (Table 1). GEP test result was class 1 in 83 (60.1%) and class 2 in 55 (39.9%) patients. Mean patient age was 60.9 years (median 62.0 years) for class 1 and 68.1 years (median 67.7 years) for class 2 tumors (P=0.002). Mean initial tumor diameter was 13.0 mm (median 13.0 mm) for class 1 and 14.1 mm (median 14.9 mm) for class 2 tumors (P=0.02). Mean initial tumor thickness was 5.2 mm (median 4.8 mm) for class 1 and 6.1 mm (median 6.0 mm) for class 2 tumors (P=0.047). Mean post-treatment tumor thickness was 3.9 mm (median 3.6 mm) for class 1 and 5.0 mm (median 4.2 mm) for class 2 tumors (P=0.002). Mean decrease in tumor thickness was 1.35 mm (median 1.0 mm) for class 1 and 1.19 mm (median 0.8 mm) for class 2 (P=0.2). Mean percent decrease in tumor thickness at 3 months after radiotherapy was 26.5% for class 1 and 16.7% for class 2 tumors (P=0.03). Four class 1 tumors, but no class 2 tumors, exhibited complete regression to a flat scar (ultrasonographic thickness = 0 mm; Figure), however this was not statistically different (P=0.16). Additionally, these regressed tumors were associated with a transient panuveitis.
Table 1.
Clinical characteristics with respect to gene expression profile classification
| Variable (Mean ± Standard Deviation) | Class 1 (n=83) | Class 2 (n=55) | Class 1 versus Class 2 (P-Value) |
|---|---|---|---|
|
| |||
| Age at Diagnosis (yrs) | 60.9 ± 13.4 | 68.1 ± 12.5 | 0.002 |
|
| |||
| Sex: | |||
| Female | 36 | 32 | 0.1 |
| Male | 47 | 23 | |
|
| |||
| Initial Tumor Largest Diameter (mm) | 13.0 ± 2.9 | 14.1 ± 2.7 | 0.02 |
|
| |||
| Initial Tumor Thickness (mm) | 5.2 ± 2.2 | 6.1 ± 2.7 | 0.047 |
|
| |||
| Tumor thickness at 3 months post-treatment (mm) | 3.9 ± 1.9 | 5.0 ± 2.3 | 0.002 |
|
| |||
| Absolute decrease in tumor thickness at 3 months post-treatment (mm) | 1.4 ± 1.4 | 1.2 ± 1.7 | 0.6 |
|
| |||
| Percent reduction in tumor thickness at 3 months post-treatment | 26.5 ± 27.2 | 16.7 ± 24.6 | 0.03 |
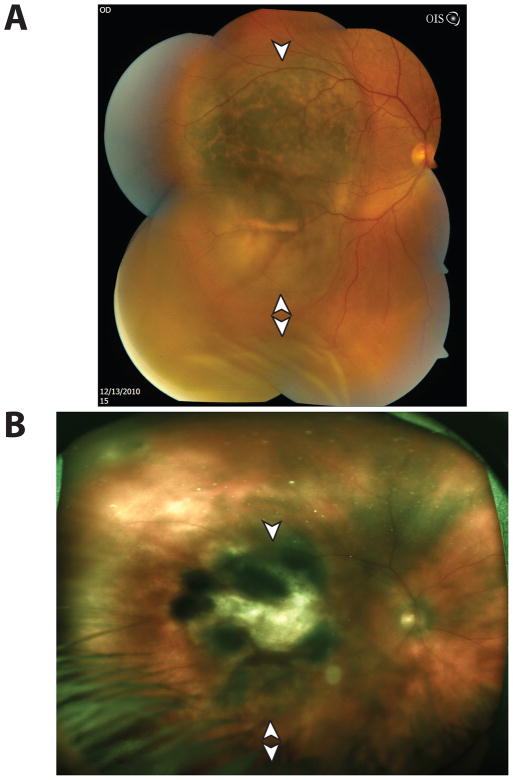
Figure 1.
Complete Regression of a Class 1 Posterior Uveal Melanoma. (A) Fundoscopic photograph of a macular uveal melanoma (arrowhead), with orange pigment and associated exudative retinal detachment inferiorly (double arrowheads). The ultrasonographic pre-treatment thickness was 2.66 mm. (B) Following episcleral plaque therapy, the tumor regressed to a flat scar (arrowhead) at 3 months as measured by B-scan ultrasonography, with resolution of the exudative retinal detachment (double arrowheads). The 3-month ultrasonographic post-treatment thickness was 0 mm. [Photo obtained 16 months following plaque therapy].
DISCUSSION
GEP class is derived from the expression of 15 genes by the melanoma.4 This profile has been shown to correlate with metastatic risk – class 1 tumors are considered low-risk while class 2 tumors are high-risk. It has been previously shown that uveal melanomas with class 1 GEP have a 2–21% risk of metastasis at 5 years while those with class 2 profile have a 72% risk of metastasis at 5 years.4 In our population, tumors with a class 1 GEP regressed more rapidly after brachytherapy than tumors with class 2 GEP. Although absolute decreases in tumor thickness were similar between the two groups, proportional decrease was greater in tumors with class 1 GEP (26.5% v. 13.0%, P = 0.01).
Interestingly, four cases of complete regression, in which the tumor regressed completely to a flat scar (three month ultrasonographic measurement at 0 mm), were present in class 1 tumors. All such cases were associated with transient panuveitis, perhaps suggesting that immune-mediated regression may account for the more rapid regression of some class 1 tumors (Figure 1). Immune-mediated regression of melanoma has been observed, yet the mechanisms remain unclear. For instance, regression of cutaneous melanoma, which develops spontaneously in a species of miniature pigs, is positively associated with acute uveitis, and is associated with migration of lymphocytes and monocytes into the stroma of the ciliary body, iris, choroid, band keratopathy, cataracts, and death of uveal melanocytes.5–7 Other studies have described enhanced regression of intraocular melanoma when melanoma cells are mutagenized in vitro prior to intraocular injection in a murine model of intraocular melanoma. Mutagenesis enhanced expression of class I major histocompatibility complex, increased susceptibility of the tumor graft to CD8+ cytotoxic T lymphocyte-mediated killing, and to tumor necrosis factor-mediated cytolysis.8 Whether certain class 1 tumors are distinctly susceptible to these particular uveitic/immunogenic-killing mechanisms remains uncertain, and deserves further study.
The five-year rate of metastasis for class 1 tumor ranges between 2–21%. Of the four patients who developed flat scars within three months during the study period, one developed metastasis (25%), approximately three months following plaque radiotherapy. However, this patient had concurrent cutaneous melanoma, and developed metastases to the lung and mediastinum, which are relatively unusual sites of metastases for primary uveal melanoma. He underwent ipilimumab therapy for late-stage cutaneous melanoma. Since the metastatic foci were not biopsied, we cannot determine whether the observed metastases originated from uveal or cutaneous sites. Even if the metastases were from the class 1 uveal melanoma, our small sample size of rapidly and completely regressed tumors, and lack of statistical significance that distinguishes the likelihood of class 1 versus 2 tumor undergoing complete regression preclude a determination of a possible link between GEP class status, metastasis and rapid and complete regression. A larger study would be helpful to further assess whether a relationship exists among these variables.
Recently, Correa et al performed a similar analysis of GEP class and post-brachytherapy regression, but found no correlation based on either absolute or proportional decrease in thickness.9 At three months, we observed a mean magnitude of regression of 1.35 mm in class 1 tumors and 1.19 mm in class 2 tumors; however, Correa et al found a mean regression of 1.3 mm in both class 1 and class 2 tumors. Their reported three-month percentage decrease in the size of class 1 tumors was similar to ours (22.4% v. 20.9%), but higher for class 2 tumors (21.0% v. 16.7%). Their study included 50 patients – 25 with class 1 and 25 with class 2 tumors whose initial tumor thicknesses were matched within 0.5mm. In our study, consistent with a previous study,10 pre-treatment thicknesses and largest basal diameters of class 2 tumors were found to be greater than class 1 tumors (Table 1, P = 0.047). In contrast to Correa et al., our report and six other studies10–15 that have calculated tumor regression rates following I-125, ruthenium-106, cobalt-60 brachytherapy, and proton beam irradiation as a function of genetic attributes (e.g. GEP class or chromosome 3 status) or likelihood of subsequent metastasis have not matched individual initial tumor thicknesses. The reason for this may be because such pair-wise matching may disproportionately exclude tumors with distinct genetic features that tend to present with thicker initial tumor thicknesses, such as tumors with class 2 status, monosomy 3, or with otherwise high propensities to metastasize.10–15 Taken together, these differences may account for some of the distinct findings observed between Correa et al. and our report.
Similar to GEP status and tumor regression following I-125 brachytherapy, the relationship among initial tumor thickness, genetic features, regression rate, metastasis, and mortality remains controversial with regard to other treatment modalities such as ruthenium-106 brachytherapy and proton beam irradiation. A previous study by one of us (J.W.H.), which included 126 patients treated with proton beam irradiation, did not find an association among the absolute magnitude of tumor thickness regression, in the velocity, or rate of thickness change, at 24 months post-treatment between class 1 and class 2 GEP. In this report however, class 1 and class 2 tumors did have a statistically significant difference in initial thickness, and tumor thickness at three months post-proton beam irradiation was not measured.10 With regard to proton beam irradiation, regression, and mortality before the era of genetic testing, Glynn et al described a more complex response relationship with proton beam irradiation: rapid initial regression during the first 2 years after irradiation portended a higher risk of metastasis.16 However, tumors that regressed slowly after the first 2 years also had a higher risk of metastasis.
In the case of ruthenium-106, Kaiserman et al. reported that tumors with the greater decrease in tumor thickness in the first three months had a higher rate of metastasis and a lower rate of survival.12 Shields et al. and Marathe et al. seemed to confirm these findings by assessing monosomy 3 status, a genetic feature of choroidal melanoma highly associated with metastasis and death, in patients with I-125 brachytherapy. Marathe et al. found that initial thickness, absolute and proportional reduction in thickness were greater among tumors with monosomy 3 (v. disomy 3, (a feature associated with increased metastasis-free survival) when measured 0.5 – 3.33 y after I-125 brachytherapy.15 Shields et al. findings were more complex: monosomy 3 was associated with a more rapid regression rate at 12 and 15 months, but, in contrast to what one would predict based on Kaiserman et al. study, monosomy 3 was not associated with more rapid regression at 4, 8, or 18 months after I-125 brachytherapy.13 Also in contrast to Kaiserman et al., Chiam et al. found that tumors with monosomy 3 did not have a significant difference in regression at six months when compared to tumors with disomy 3.14
The relationship between response to brachytherapy and metastasis and survival has been studied even before the advent of genetic profiling. Augsburger et al showed that rapid regression of choroidal melanoma following cobalt-60 brachytherapy is associated with lower metastatic-free survival.17 Glynn et al described a more complex response relationship with proton beam irradiation: rapid initial regression during the first 2 years after irradiation portended a higher risk of metastasis. However, tumors which regressed slowly after the first 2 years also had a higher risk of metastasis.16 In summary, previous studies relating tumor regression, genetic features, metastasis and survival remain conflicting and do not arrive at a consensus.
While our report arrives at conclusions distinct from the conflicting previous studies, our study has important limitations, including relatively small sample size and retrospective study design. The follow-up time was short, but this was by design, as our goal was to analyze early tumor response following brachytherapy. As a result of this short follow-up time, we are unable to identify any relationship between response of brachytherapy and metastasis, precluding a determination of whether response to brachytherapy can predict tumor metastasis within the class 1 or class 2 tumors. Interestingly, four of the class 1 tumors, but no class 2 tumors, exhibited rapid regression to a flat scar at three months by ultrasonography. While these cases of complete regression in class 1 may appear to be outliers, statistical analysis of all cases in the GEP class 1 dataset indicates that all Z-scores (representing individual percentage changes in tumor thicknesses subtracted from the mean percentage change, with the corresponding difference divided by the standard deviation) fall within −3 to +3. Thus, the cases of complete regression in class 1 are unlikely to represent outliers and were not excluded from analysis.
In conclusion, we demonstrate that class 1 tumors proportionally regress more than class 2. Complete regression at 3 months was observed only among class 1 cases.
Acknowledgments
This work was supported by grants to J.W.H. from the National Cancer Institute (R01CA125970), Melanoma Research Foundation, Melanoma Research Alliance and the Tumori Foundation. R.C.R. was supported by the National Eye Institute (K12EY022299) and by a merit award from Heed Ophthalmic Foundation and Society of Heed Fellows.
Footnotes
Financial Disclosure: Dr. Harbour is the inventor of intellectual property used in the study and receives royalties from its commercialization. He is a paid consultant for Castle Biosciences, licensee of intellectual property presented in this article.
Contributions of Authors: Conception and design (RCR, SNB, JWH), data acquisition (RCR, SNB, JWH), analysis and interpretation (RCR, MK, SNB, JWH), drafting the manuscript (RCR, JWH), revision of the manuscript (RCR, MK, SNB, JWH), final approval of the manuscript (JWH).
References
- 1.Ramaiya KJ, Harbour JW. Current management of uveal melanoma. Expert Review of Ophthalmology. 2007;2(6):939–46. [Google Scholar]
- 2.Onken MD, Worley LA, Ehlers JP, Harbour JW. Gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res. 2004;64:7205–9. doi: 10.1158/0008-5472.CAN-04-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Onken MD, Worley LA, Char DH, et al. Collaborative Ocular Oncology Group report number 1: prospective validation of a multi-gene prognostic assay in uveal melanoma. Ophthalmology. 2012;119(8):1596–603. doi: 10.1016/j.ophtha.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Field MG, Harbour JW. Recent developments in prognostic and predictive testing in uveal melanoma. Curr Opin Ophthalmol. 2014;25(3):234–9. doi: 10.1097/ICU.0000000000000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lentz KJ, Burns RP, Loeffler K, et al. Uveitis caused by cytotoxic immune response to cutaneous malignant melanoma in swine: destruction of uveal melanocytes during tumor regression. Invest Ophthalmol Vis Sci. 1983;24(8):1063–9. [PubMed] [Google Scholar]
- 6.Feeney-Burns L, Burns RP, Gao CL. Ocular pathology in melanomatous Sinclair miniature swine. Am J Pathol. 1988;131(1):62–72. [PMC free article] [PubMed] [Google Scholar]
- 7.Richerson JT, Burns RP, Misfeldt ML. Association of uveal melanocyte destruction in melanoma-bearing swine with large granular lymphocyte cells. Invest Ophthalmol Vis Sci. 1989;30(12):2455–60. [PubMed] [Google Scholar]
- 8.Knisely TL, Niederkorn JY. Immunologic evaluation of spontaneous regression of an intraocular murine melanoma. Invest Ophthalmol Vis Sci. 1990;31(2):247–57. [PubMed] [Google Scholar]
- 9.Correa ZM, Augsburger JJ. Relationship between rate of posterior uveal melanoma flattening following plaque radiotherapy and gene expression profile class of tumor cells. Invest Ophthalmol Vis Sci. 2014;55(1):556–9. doi: 10.1167/iovs.13-13381. [DOI] [PubMed] [Google Scholar]
- 10.Chappell MC, Char DH, Cole TB, et al. Uveal melanoma: molecular pattern, clinical features, and radiation response. Am J Ophthalmol. 2012;154(2):227–32. e2. doi: 10.1016/j.ajo.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruess AF, Augsburger JJ, Shields JA, et al. Regression of posterior uveal melanomas following cobalt-60 plaque radiotherapy. Ophthalmology. 1984;91(12):1716–9. doi: 10.1016/s0161-6420(84)34087-8. [DOI] [PubMed] [Google Scholar]
- 12.Kaiserman I, Anteby I, Chowers I, et al. Post-brachytherapy initial tumour regression rate correlates with metastatic spread in posterior uveal melanoma. Br J Ophthalmol. 2004;88(7):892–5. doi: 10.1136/bjo.2003.036285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shields CL, Bianciotto C, Rudich D, et al. Regression of uveal melanoma after plaque radiotherapy and thermotherapy based on chromosome 3 status. Retina. 2008;28(9):1289–95. doi: 10.1097/IAE.0b013e31817f7b3e. [DOI] [PubMed] [Google Scholar]
- 14.Chiam PJ, Coupland SE, Kalirai H, et al. Does choroidal melanoma regression correlate with chromosome 3 loss after ruthenium brachytherapy? Br J Ophthalmol. 2014 doi: 10.1136/bjophthalmol-2013-304472. [DOI] [PubMed] [Google Scholar]
- 15.Marathe OS, Wu J, Lee SP, et al. Ocular response of choroidal melanoma with monosomy 3 versus disomy 3 after iodine-125 brachytherapy. Int J Radiat Oncol Biol Phys. 2011;81(4):1046–8. doi: 10.1016/j.ijrobp.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 16.Glynn RJ, Seddon JM, Gragoudas ES, et al. Evaluation of tumor regression and other prognostic factors for early and late metastasis after proton irradiation of uveal melanoma. Ophthalmology. 1989;96(10):1566–73. doi: 10.1016/s0161-6420(89)32685-6. [DOI] [PubMed] [Google Scholar]
- 17.Augsburger JJ, Gamel JW, Shields JA, et al. Post-irradiation regression of choroidal melanomas as a risk factor for death from metastatic disease. Ophthalmology. 1987;94(9):1173–7. doi: 10.1016/s0161-6420(87)33310-x. [DOI] [PubMed] [Google Scholar]