Figure 1.
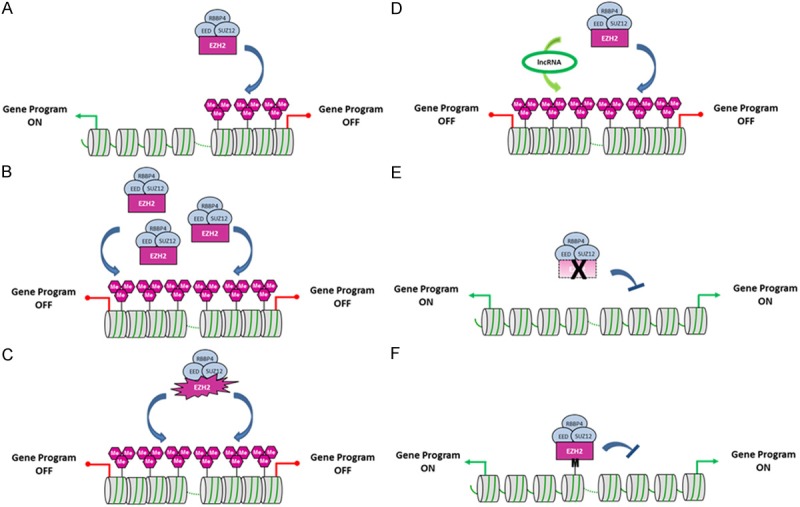
Schematic representation of the diverse EZH2 dysregulations found in cancer. A. The histone lysine methyltransferase EZH2 catalyzes H3K27 methylation at defined target genes and silences their expression. B. Overabundance of EZH2 is responsible for an increase in H3K27me3 repressive mark levels leading to the silencing of tumor suppressor genes in cancer cells. C. EZH2 bearing activating mutations at residues Y641, A677 or A687 possesses an enhanced activity leading to an increase in H3K27me3 levels. D. Overexpression of EZH2-interacting partners such as specific lncRNAs enhances recruitment of EZH2 to targets and increases H3K27me3 levels. E. EZH2 harboring an inactivating mutation or EZH2 gene deletion leads to a decrease in H3K27me3 levels and activation of EZH2 target gene programs in cancer. F. A lysine to methionine substitution at position 27 (K27M) in the gene encoding histone H3.3 (H3F3A) inhibits EZH2 activity and leads to nearly undetectable H3K27me3 repressive mark levels in pediatric gliomas. Purple hexagons represent H3K27me epigenetic marks, and M illustrates H3.3K27M mutant histones.
