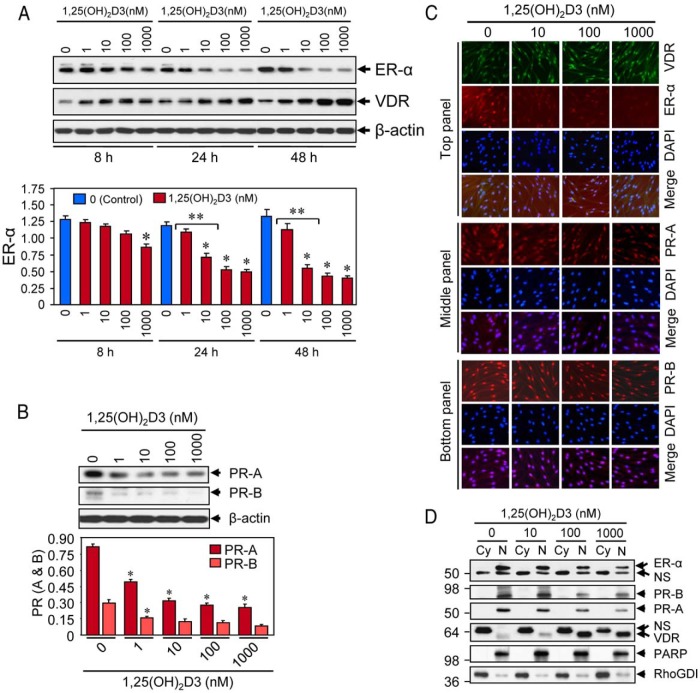
Figure 3.
Effect of 1,25(OH)2D3 on reduction of sex steroid receptors in cultured HuLM cells. A, HuLM cells were serum starved and treated with increasing doses of 1,25(OH)2D3 (0, 1, 10, 100, and 1000 nm) for 8, 24, and 48 hours, and then protein lysates were prepared. Equal amounts of each protein lysate were analyzed by Western blot using anti-ER-α and anti-VDR antibodies. Western blot with anti-β-actin antibody was used as the loading control (top panel). The intensities of ER-α protein bands were quantified and normalized to β-actin, and relative values were used to generate graphic data (bottom panel). *, P < .05, as compared to the corresponding control. **, P < .05 indicates the significance between consecutive doses. VDR and β-actin data have been published previously (19). B, Above HuLM cell lysates were also analyzed for protein expression of PR-A and PR-B. Western blot with anti-β-actin antibody was used as the loading control (top panel). The intensity of each protein band was quantified and normalized to β-actin, as indicated above. *, P < .05 as compared to the corresponding control (bottom panel). C, Immunofluorescence analyses. HuLM cells were cultured on glass cover slips, serum starved for 20 hours, and treated with increasing doses of 1,25(OH)2D3 (0, 10, 100, and 1000 nm) for 48 hours. Cells were fixed in 3.7% formalin solution, permeabilized in 0.2% Triton X-100/PBS, and then incubated with monoclonal anti-ER-α (top panel), or polyclonal anti-VDR (top panel), or anti-PR-A (middle panel), or anti-PR-B (bottom panel) antibodies (1: 50 dilution each) followed by incubation with CY3-conjugated (red) or FITC-conjugated secondary antibodies as appropriate. Nuclei of cells were monitored by 4′,6 diamidino-2-phenylindole (DAPI) (blue). Pictures were captured at ×200 magnification. D, HuLM cells were serum starved and treated with increasing doses of 1,25(OH)2D3 (0, 10, 100, and 1000 nm) for 48 hours. Cytoplasmic (Cy) and nuclear (N) fractions were prepared from 1,25(OH)2D3-treated cells. Equal amounts of each fraction (15 μg) were subjected to Western blot analyses using anti-ER-α, anti-PR-A, anti-PR-B, and anti-VDR antibodies. Western blot with anti-PARP (a nuclear protein) and anti-RhoGDI (a cytoplasmic protein) antibodies were used to ensure the extraction efficiency. The expression of PARP was almost exclusively localized in the nuclear fractions, whereas the expression of RhoGDI was almost exclusively localized in the cytosolic fractions. NS, nonspecific.