Abstract
Recent studies have shown that monocytes and macrophages not only present antigens to effector T cells and stimulate and shape T cell-mediated immune responses, but they also prime naïve T cells, thus initiating adaptive immune responses. Phosphatidylinositol 3-kinase functions at an early phase of toll-like receptor signaling pathways, modulates the magnitude of the primary immune responses, and is involved in the reorganization of the actin cytoskeleton during macropinocytic and phagocytic antigen uptakes, important early steps in triggering adaptive immune responses.
We assessed by flow cytometry the endocytic capacities of bovine monocytes by using endocytic tracers and Salmonella transformed with a green fluorescence plasmid GFP to evaluate macropinocytosis, mannose receptor-mediated endocytosis, and phagocytosis in bovine professional antigen presenting cells, respectively. Our data reveal that wortmannin, an inhibitor of phosphatidylinositol 3-kinase signaling pathway, significantly increased macropinocytosis and phagocytosis but did not affect the mannose receptor-mediated antigen uptake in bovine monocytes. Protein expression data support these findings by showing decreased levels of phosphoinositide 3-kinase in the presence of wortmannin during macropinocytosis.
We expanded further the key role of phosphatidylinositol 3-kinase as an endogenous suppressor of primary immune responses, suggesting a novel mechanism of phosphatidylinositol 3-kinase antigen uptake modulation that may provide a unique therapeutic target for controlling excessive inflammation.
Keywords: Phosphatidylinositol 3-Kinase Pathway, Wortmannin, Monocytes, Phagocytosis, Macropinocytosis, Receptor-Mediated Endocytosis, Antigen Uptake
Introduction
Recent studies show that monocytes and macrophages not only present antigens to effector T cells and stimulate and shape T cell-mediated immune responses, but they also prime naïve T cells, thus initiating adaptive immune responses [1–3]. In particular, monocytes are recruited rapidly to the site of infection, they give rise to macrophages and inflammatory dendritic cells (DCs), are recruited directly from the blood via high endothelial venules to the T cell areas of the lymph nodes and finally, are able to cross-present antigens derived from endocytosed pathogens to cytotoxic T cells [2]. Monocytes and macrophages express multiple phagocytic and signaling pattern recognition receptors (PRRs) that sense and bind pathogen-associated molecular patterns (PAMPs). Namely, C-type lectin mannose receptor (MR) and toll-like receptors (TLRs) are involved in bacterial pathogen uptake in the early phase of infection, whereas receptors that bind antigens opsonized with IgG, mostly Fc receptors, scavenger receptors, and activated complement factors are involved in later stages when monocytes differentiate into macrophages [4, 5]. The glycoprotein ovalbumin (OVA), which contains mannose residues, was reported to be endocytosed through the MR, that has been shown to be essential for pro- and anti- inflammatory cytokine production and is dependent on TLR-triggering by pathogens or synthetic ligands [6, 7].
Macropinocytosis is a potent non-selective mechanism of antigen uptake limited to immature DCs and their myeloid progenitors, monocytes and macrophages. Activated by exogenous stimuli, internalization of solutes via macropinocytosis provides a unique endocytic pathway to complement immune monitoring via PRRs [8, 9]. The internalization of solutes by macropinocytosis is much more effective than by other non-selective mechanisms [8, 9]. Phagocytosis also is an important early step in triggering the adaptive immune responses, which require processing of bacterial pathogens and presentation of their antigens to CD4+ and CD8+ T cells by professional antigen presenting cells (APCs) [2].
Macropinocytosis and phagocytosis depend on membrane-cytoskeleton interactions, thereby reflecting cortical actin dynamics and cell motility, and phosphatidylinositol 3-Kinase (PI3K) activation was demonstrated to be involved in the dramatic reorganization of the actin cytoskeleton [10]. One of the most potent inflammatory agents able to activate monocytes and macrophages is LPS, a component of the outer membrane of Gram-negative bacteria that binds to the surface protein CD14 and stimulates the TLR4-MD2 complex that results in rapid production of inflammatory mediators and cytokines in monocytes and macrophages. Namely, IL-1α, IL-1β, IL-6, IL-12 and TNF-α are produced mainly through the activation of the NF-kB and mitogen-activated protein kinase (MAPK) signaling pathway [11]. Previously, several reports showed the involvement of MAPK signaling pathway in LPS/E. coli active phagocytosis in medfly hemocytes and in West Nile virus endocytosis, including phagocytosis, in a mosquito cell line [12–14].
The resultant inflammatory response is essential for the eradication of pathogens; however, excessive and prolonged activation of innate immunity is harmful, and, in some cases, even fatal to the host, owing to severe tissue damage and circulatory failure [15]. Several studies have shown that PI3K is an endogenous suppressor of IL-12 and IL-1β production triggered by TLR signaling and limits excessive Th1 type polarization [16–20]. Unlike other gate-keeping systems, IRAK–M (IL-1 receptor-associated kinase-M) and SOCS-1 (suppressor of cytokine signaling-1) induced by TLR signaling and function during the second or continuous exposure to stimulation, PI3K functions at the early phase of TLR signaling, modulates the magnitude of primary activation, and thus has an early, unique role in the gate-keeping system, preventing excessive innate immune responses [17].
Salmonella enterica, with over 2000 different serovars, is indigenous to the gastrointestinal tracts of many mammals, birds, and reptiles, usually at low levels [21]. Salmonella is hagocytosed by monocytes/macrophages and DCs, in which it replicates triggers rapid tissue destruction and inflammation [22]. Apoptosis of macrophages in the liver occurs during systematic Salmonella infection in vivo. In vitro Salmonella strains induced delayed apoptosis that requires activation of TLR4 on macrophages by the bacterial LPS [23]. NF-κB and MAPK are particularly important for the induction of anti-apoptotic factors [23]. Salmonella virulence proteins are essential for altering the balance in favor of apoptosis during intracellular infection, but mechanisms involved are not understood fully.
Despite numerous observations that have implicated PI3K signaling as a regulator of various biological functions, including the pro-inflammatory response to TLR signaling, PI3K effects on inflammatory response varies, depending on several factors that remain to be elucidated [24, 25].
In this study, we investigated the role of PI3K in the early stages of an immune response, antigen uptake mediated via macropinocytosis, MR-mediated endocytosis, and phagocytosis in bovine monocytes. We hypothesized that PI3K plays an important role as an endogenous regulator of the TLR-dependent and independent signaling cascades initiated during macropinocytosis and phagocytosis in bovine monocytes.
Materials and methods
Animals
Conventionally reared, healthy cows from a Holstein herd at the Mississippi State University Dairy Facility were used. The animals have been subjected to a comprehensive vaccination program, including Frontier 4 Plus Vaccine (IBR, BVD, PI3, RSV, Diamond Animal H, Inc). The Mississippi State University Institutional Animal Care and Use Committee approved all animal use (IACUC #09-039).
Cell Preparation
Bovine peripheral blood mononuclear cells (PBMCs) were separated as described elsewhere [33, 34]. Briefly, PBMCs were isolated on Histopaque gradients (1.077 g/ml, Sigma). Cells were resuspended to 5×106 per ml in RPMI-1640 supplemented with 10% FBS, 1% Glutamax-1 (Gibco Life Technologies), 5 × 10−5 M 2-mercaptoethanol, 75 μg/ml gentamicin (Gibco Life Technologies).
To isolate monocytes, PBMCs were added to a tissue culture plate for 10–12 hours at 37° C. After removing non-adherent cells, the adherent cells (70–80% monocytes) were washed twice in PBS and incubated with endocytic tracers.
Salmonella preparation and infection
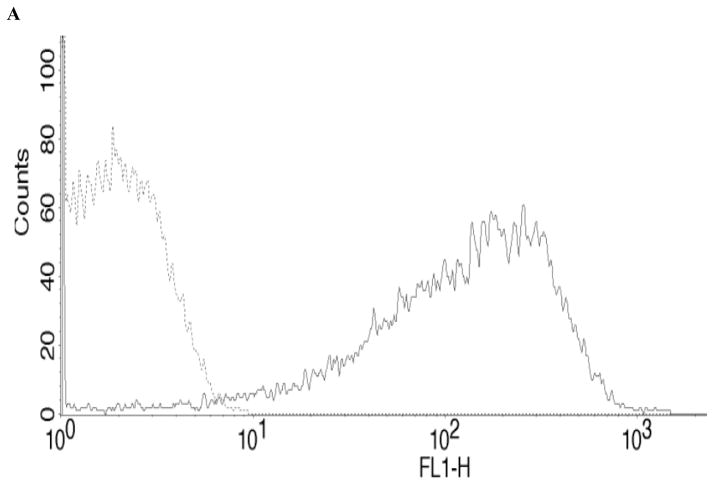
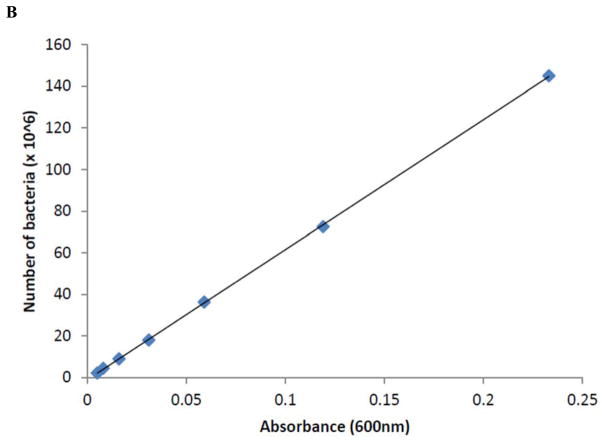
Salmonella kentucky strain was isolated from chickens and serotyped at Poultry Research and Diagnostics Laboratory (Jackson, CVM-MSU). This strain was transformed with GFP, a plasmid with green fluorescence, as described previously [49]. Salmonella kentucky was cultured overnight in LB with ampicillin at 37° C and centrifuged at 3000xg. GFP expression was confirmed by flow cytometry (Fig. 1A), and a bacterial growth standard curve determined by spectrophotometry [Genesys 20, Thermo Scientific] (Fig. 1B). To opsonize S. kentucky, the bacterial strain was cultured in the presence of 10% BSA. We used heat-inactivated (HI) S. kentucky to evaluate the scavenger-receptor-mediated phagocytosis in bovine monocytes, as described [50]. Briefly, S. kentucky at concentration 108 bacterial cells/ml was HI at 60° C for 30 min. To confirm that bacterial cultures were sterile, S. kentucky was cultured in LB with ampicillin overnight at 37° C.
Figure 1.
Fluorescence intensity and bacterial growth of S. kentucky.
(A) - Fluorescence intensity of GFP-labeled (GFP+) S. kentucky.
GFP+ S. kentucky (bold solid line)
GFP− S. kentucky (dotted line)
(B) - S. kentucky bacterial growth standard curve by spectrophotometry.
Endocytosis Assays and Flow Cytometry
The endocytic capacities of monocytes were determined by using following endocytic tracers: Lucifer Yellow (LY) and fluorescein isothiocyanante-labeled ovalbumin (FITC-OVA) (both from Invitrogen) to evaluate macropinocytosis and MR-mediated endocytosis, respectively.
The ability of monocytes to endocytose FITC-OVA and LY was measured as described elsewhere [33, 34, 42]. Briefly, monocytes were treated with FITC-OVA or LY at final concentrations 100 μg/ml for 30 min at 37° C to measure active endocytosis or at 4° C to determine background levels of endocytosis (negative control). Monocytes were washed three times by centrifugation in cold PBS and analyzed using a FACSCalibur (Becton Dickinson) as follows. After setting a gate on large granular cells, the FITC-OVA or LY incorporation was measured and analyzed. To inhibit selectively various pathways involved in antigen uptake, bovine monocytes were incubated for 15 min in the presence of cytochalasin D [CCD] (2.5 μg/ml), latrunculin A [5μM] and wortmannin [5–20 μM] (all from Sigma) before the addition of FITC-OVA or LY.
To evaluate S. kentucky phagocytosis in bovine APCs, 108 live, HI and BSA-treated bacterial cells were added for 2 hours to monocyte cultures and incubated at 37° C. To determine background levels of phagocytosis (negative controls), cells were incubated in the presence of S. kentucky at 4° C. Cells were washed three times by centrifugation in cold PBS and analyzed by flow cytometry.
Western blot
Kinase p85 expression levels were determined by Western blotting analysis. Adherent PBMC populations (70–80% pure monocytes) were treated with FITC-OVA or LY at final concentrations 100 μg/ml for 30 min at 37 °C. To inhibit selectively PI3K pathway involved in antigen uptake, cells were incubated for 15 min in the presence of wortmannin [5 μM] before addition of FITC-OVA or LY. Cells were washed three times by centrifugation in cold PBS, and monocyte protein extractions for all treatments were performed using RIPA Buffer (Thermo Scientific). The concentration of proteins were then determined using a BCA Protein Assay Kit (Thermo Scientific) and 45 μg samples loaded on a 8–16% gradient Precise Protein Gel (Thermo Scientific), for SDS-PAGE. The proteins were then transferred to Immun-Blot PVDF (BIO-RAD) for detection. The blots were probed with the following primary antibodies: anti-PI3 Kinase (LifeSpan BioSciences), anti-MAP Kinase 1,2 (USBiological), and the housekeeping protein anti-β-actin (Ambion). Anti-PI3 kinase (p85 regulatory subunit) and anti-β-actin antibodies were then labeled with goat anti-mouse IgG (H+L)-AP (Zymed) secondary antibody, and the MAP kinase 1,2 antibody was labeled with goat anti-rabbit IgG (H+L)-AP (Invitrogen) secondary antibody. The labeled proteins were detected with BCIP/NBT Phosphatase Substrate (KPL).
Data analysis
Data (2–7 independent experiments) were analyzed by analysis of variance (ANOVA) followed by Fisher’s LSD multiple comparison post hoc test and are presented as means + SD. The level of significance for all tests of effects was set as P<0.05.
Results
Effect of Wortmannin on the Endocytosis in Bovine Moncytes
We demonstrated previouslythat bovine monocytes cultured for up to 24 hrs had moderate capacity to uptake FITC-DX and low to moderate capacity to endocytose LY, suggesting that macropinocytosis did not play a significant role in active antigen uptake in bovine monocytes [26]. To determine if the early phases of selective and non-selective antigen uptake in monocytes are regulated by PI3K-dependant signaling pathways, we evaluated the effect of PI3K inhibitor, wortmannin (W), on antigen uptake by phagocytosis, MR-mediated antigen uptake and macropinocytosis in bovine monocytes.
In this study, bovine PBMCs were cultured for 10–12 hrs, and adherent cells, 70–80% pure monocytes, were collected to assess the cells ability to endocytosis with following endocytic tracers LY, FITC-OVA and live, opsonized and HI S. kentucky. To assess macropinocytosis in bovine APCs, we measured the uptake of LY in cells pre-incubated with the inhibitors of macropinocytosis, CCD, latrunculin A (LAT), the PI3K inhibitor, W, and control cells in medium only. To ensure active endocytosis in monocytes, their viability in the presence of the inhibitors was assessed by flow cytometry. Inhibitors, W and CCD, did not change the numbers, granularity, or size of monocytes in the designated region at all concentrations used which varied from 72% to 79% of total PBMCs compared to the 37° C (74% of total PBMCs) and 4° C (70% of total PBMCs) controls (data not shown). However, LAT at concentration 100 μM promoted apoptosis in bovine monocytes as seen by significant changes in cell size and granularity and decreased numbers of APCs in the designated region to 12% of total PBMCs (data not shown). As expected, monocytes after 12 hrs culture expressed an insignificant capacity to uptake LY that decreased numerically in the presence of CCD and LAT (Fig. 2). However, the uptake of LY was enhanced significantly in the presence of W (Fig. 2). The significant increases in LY uptake were evident in the presence of W at all concentrations used; however, after 2 hrs of W exposure the uptake of LY was inhibited completely in bovine monocytes (data not shown).
Figure 2.
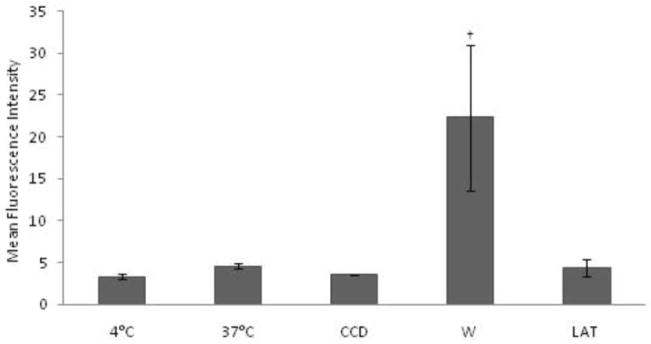
Macropinocytosis of LY in bovine monocytes. LY uptake at 37° C was assessed in monocytes by flow cytometry in the presence or absence of the macropinocytosis inhibitors CCD [2.5 μg/ml], LAT [5μM] and W [5μM]. Macropinocytosis at 4° C was measured to determine background levels of LY uptake. Samples were analyzed in two representative experiments, and data are expressed as Mean Fluorescent Intensity (MFI). † Presence on the top of bars indicate treatment differences from bars without † designation (P < 0.05).
To characterize the possible role of PI3K in receptor-mediated endocytosis in monocytes, the uptake of FITC-OVA in control cells and APCs pre-incubated in the presence of CCD and W has been analyzed. Bovine monocytes actively endocytosed FITC-OVA at 37° C, and this uptake was inhibited significantly in the presence of CCD (Fig. 3). However, bovine APCs did not show significant increases in OVA endocytosis in the presence of W compared to antigen uptake at 37° C (Fig. 3).
Figure 3.
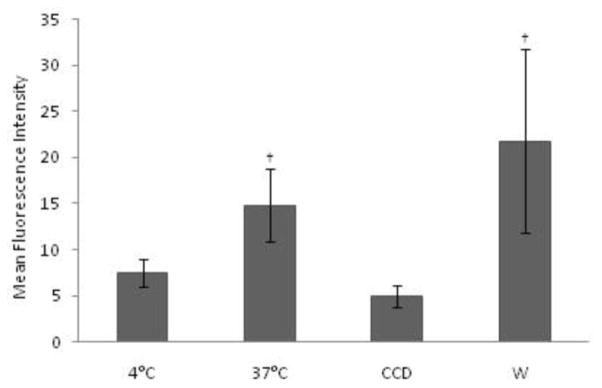
Endocytosis of FITC-OVA in bovine monocytes. FITC-OVA endocytosis was assessed in bovine monocytes by flow cytometry in the presence or absence of endocytosis inhibitor CCD [2.5 μg/ml] and W [5μM]. Endocytosis at 4° C was measured to determine background levels of FITC-OVA uptake. Samples were analyzed in seven representative experiments, and data are expressed as Mean Fluorescence Intensity (MFI). † Presence on the top of bars indicate treatment differences from bars without † designation (P < 0.05).
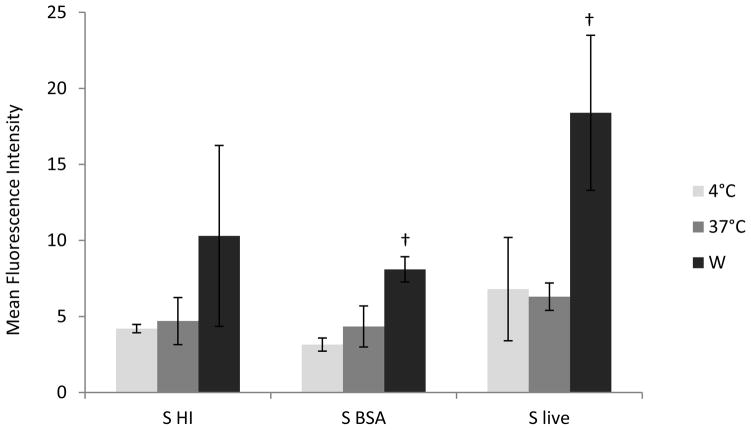
Finally, to investigate the role of the PI3K pathway inhibitor W in the phagocytic antigen uptake in bovine monocytes, we used as an antigen live, opsonized and inactivated by exposure to high temperature (HI) S. kentucky. Monocytes after 2 hrs incubation with live S. kentucky at 37° C did not express active phagocytic ability; however, consistent, non-significant increases in bacterial antigen uptake of opsonized and HI S. kentucky have been observed (Fig. 4). The addition of W promoted enhanced significantly phagocytosis of live and opsonized S. kentucky in bovine monocytes (Fig. 4). Phagocytosis of HI bacterial cells was non-significantly increased in the presence of W [p = 0.06] (Fig. 4).
Figure 4.
Phagocytosis of GFP-labeled Salmonella Kentucky in bovine monocytes. Antigen uptake by phagocytosis was evaluated in bovine monocytes in the presence or absence of W [5μM]. Phagocytosis at 4° C was measured to determine background levels of S. kentucky uptake. Samples were analyzed in three (S. live), three (S. BSA or opsonized) and four (S. HI) representative experiments, and data are expressed as Mean Fluorescence Intensity (MFI). † Presence on the top of bars indicate treatment differences from bars without † designation (P < 0.05).
Phosphoinositide 3-kinase protein expression in selective and non-selective endocytosis in bovine monocytes
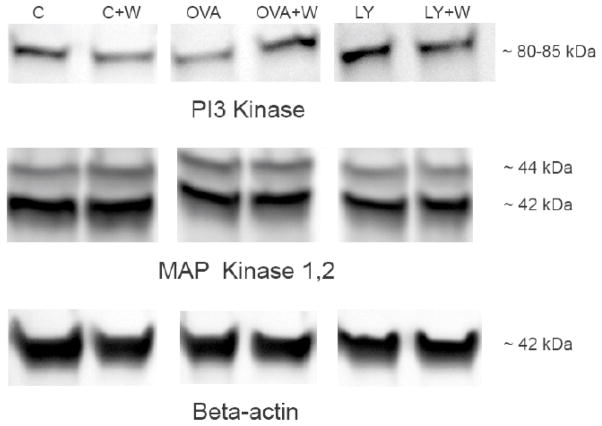
To evaluate further the involvement of PI3K- and MAPK- mediated pathways in active endocytic antigen uptake in bovine monocytes, we assessed by Western blot analysis kinase proteins expression levels. Kinase protein data revealed that PI3K and MAPK are expressed in bovine monocytes in the absence of OVA and LY. The protein expression data show that the regulatory subunit of PI3K (p85) protein visual expression levels in bovine monocytes have been decreased in the presence of OVA and increased in monocytes incubated with LY compared to control cells (Fig. 5). The addition of W inhibited the PI3K expression in control monocytes and eliminated the effects of OVA and LY on the expression levels of PI3K in bovine monocytes (Fig. 5). Expression levels of MAPK and β-actin proteins were unchanged in the presence of W in all three experimental groups (Fig. 5).
Figure 5.
Western blot analysis of PI3K and MAPK protein expression in the presence of PI3K inhibitor in bovine monocytes relative to that of control monocytes with β-actin as housekeeping protein. One of two representative experiments.
Discussion
Investigations into the actin cytoskeleton regulatory signaling events that may be involved in the endocytic pathways are limited in professional APCs. PI3K has been implicated in the regulation of actin-dependent endocytosis, intracellular membrane traffic, and cell growth [27]. Similarities between the signaling and mechanical pathways used in phagocytosis and macropinocytosis often imply that inhibitors block both pathways, as is the case with PI3K inhibitors, in particular W [27, 28]. Several earlier reports demonstrated that W blocks both macropinocytosis and FcR-mediated phagocytosis in murine bone marrow-derived macrophages, transformed fibroblasts and macropinocytosis in murine immature DCs by binding PI3K catalytic subunit and irreversibly inhibits the regulatory subunit [10, 27–30]. In contrast to previous observations in our study, W enhanced significantly macropinocytosis and the phagocytic uptake of live and opsonized S. kentucky in bovine monocytes, suggesting the negative regulatory role for PI3K in monocyte antigen uptake. Importantly, our data agree and contribute to the recent reports on the PI3K-mediated negative regulation of multiple innate immune responses, including IL-12, IL-1β production and Th1 polarization defining PI3K as a negative regulator in the early phase of the innate immune responses [17–20, 31, 32]. In this study, we show that the negative signaling regulation by PI3K is involved in the important professional APC function of monocytes, non-selective and selective antigen uptakes.
Differences From the earlier observations regarding W effects on the actin-mediated antigen uptake mechanisms in our study could be due to several reasons. Firstly, there are phenotypic and functional differences between monocytes as myeloid progenitor cell populations and fully differentiated cells such as macrophages, fibroblasts, and DCs. Secondly, there are some species-specific differences in the APCs functions [33, 34]. Finally, different experimental conditions and difficulties in studying the role of PI3K in TLR signaling [24] explain the differences in the effects of W on endocytic antigen uptake. Our results revealed that bovine monocytes incubated with W for 2 hrs and longer expressed significant dose-dependent decreases in non-selective, fluid phase uptake and phagocytosis in the presence of W, thus confirming previous reports on the inhibitory effects of W in the actin-mediated endocytic uptake [10, 27–30].
In our study, we demonstrated that W did not alter significantly active endocytosis of FITC-OVA in bovine monocytes. Active endocytosis of FITC-OVA in bovine monocytes was inhibited significantly in the presence of actin polymerization inhibitor CCD, suggesting that macropinocytosis was the major mechanism of the OVA uptake [8]. This finding does not contradict previous data on the inhibitory effects of W on both macropinocytosis and FcR-mediated phagocytosis [10, 27, 28, 35] because endocytosis of OVA is not FcR-mediated, not restricted exclusively to macropinocytosis, and is conducted through MR-mediated and/or macropinocytic mechanisms [4, 5, 36].
In contrast to significant OVA endocytosis, active antigen uptake of the live, opsonized or HI S. kentucky was not observed in bovine monocytes incubated in the presence of bacteria for 2 hrs. Our findings are in agreement with previous reports demonstrating that TLRs are involved in bacterial, including some highly pathogenic Salmonella strains, uptake in the early phase of infection, whereas receptors that bind opsonized antigens, mostly Fc receptors, are involved in later stage when monocytes differentiate into macrophages [4, 5, 23, 37].
In this study, protein expression levels of MAPK, the kinase was demonstrated to “share” the TLR-induced signaling pathways with PI3K [38], did not change visually in the presence of OVA, LY and W in bovine monocytes, suggesting that the PI3K signaling was TLR-independent.
According to the earlier reports, the PI3K protein expression showed decreases at very low nanomolar concentrations of W, and the complete inhibition of PI3K was reached at higher concentrations of 0.1 to 10 μM in finally differentiated cells [10, 27, 28, 30, 35, 39]. Our data agree with previous observations that PI3K is expressed constitutively in innate immune cells [17, 31, 32, 40]. However, in contrast to the earlier reports that PI3K is activated rapidly by antigens, expression levels of PI3K have been decreased in the presence of OVA, suggesting that OVA antigen, unlike some pathogens, does not involve PI3K-dependant TLR signaling pathways [17, 31, 32, 40]. Interestingly, the decreased levels of PI3K in the presence of OVA correlate with significantly increased active OVA uptake in bovine monocytes, thus supporting our hypothesis on the role of PI3K as an endogenous regulator of antigen uptake in professional APCs. The addition of LY increased the expression levels of PI3K, and W eliminated this increase in monocytes incubated with LY. Interestingly, decreased PI3K levels correlated with the significantly enhanced macropinocytosis in bovine APCs in the presence of the PI3K inhibitor W. This finding suggests that PI3K is an endogenous suppressor of signaling events involved in macropinocytosis that is not receptor-mediated but not in the MR-mediated uptake of OVA in bovine monocytes.
Conclusion
In conclusion, we expanded further the key role of PI3K as an endogenous suppressor in the TLR and non- TLR-dependant signaling cascades during macropinocytosis and phagocytosis in bovine monocytes. In light of emerging evidence on the plasticity of monocytes responding to their environment by differentiating into a variety of macrophages and DC-like cells [41], the regulatory signaling events that control early antigen uptake mechanisms are especially important. Viruses and other pathogens have subverted macropinocytosis and phagocytosis by activating signaling pathways, including PI3K-dependent that trigger actin-mediated membrane ruffling and blebbing [27, 42–46], and some viruses use other endocytic mechanisms for entry but require macropinocytosis to promote penetration [47, 48]. Therefore, the aspect of emerging importance is to investigate further the molecular signaling control of lineage commitment in the mononuclear phagocyte system.
Collectively, the unique features of phosphoinositide 3-kinase as an endogenous suppressor of primary immune responses and its contributions to regulation of antigen uptake in bovine monocytes may provide a unique therapeutic target for controlling excessive inflammation. Thus, the better understanding of how immunity is regulated via PI3K, would allow us to manipulate those signals and in this way, develop strategies to modulate, prevent, and treat the multiple diseases in which this mechanism is implicated
Acknowledgments
We acknowledge the assistance of Ryan Lawrence (Lab Assistant) in the animal bleeding and PBMC separation procedures. We also thank Dr. John Harkness for editing this manuscript.
Abbreviations
- DCs
dendritic cells
- PRRs
pattern recognition receptors
- PAMPs
pathogen-associated molecular patterns
- MR
mannose receptor
- TLRs
toll-like receptors
- OVA
ovalbumin
- APCs
antigen presenting cells
- PI3K
Phosphatidilinositol 3-Kinase
- LPS
lypopolysaccharide
- MAPK
mitogen-activated kinase
- IRAK-M
IL-1 receptor-associated kinase-M
- SOCS-1
suppressor of cytokine signalling-1
- PBMCs
peripheral blood mononuclear cells
- S
Salmonella
- GFP
green fluorescence plasmid
- LY
Lucifer Yellow
- FITC-OVA
fluorescein isothiocyanante-labelled ovalbumin
- CCD
cytochalasin D
- W
wortmannin
- HI
heat-inactivated
Footnotes
Conflict of Interest
No financial and competing interests are declared
References
- 1.Olazabal IM, Martin-Cofreces NB, Mittelbrunn M, Martinez del Hoyo G, Alarcon B, Sanchez-Madrid F. Activation outcomes induced in naive CD8 T-cells by macrophages primed via “phagocytic” and nonphagocytic pathways. Mol Biol Cell. 2008;19:701–710. doi: 10.1091/mbc.E07-07-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geissmann F, Auffray C, Palframan R, Wirrig C, Ciocca A, Campisi L, Narni-Mancinelli E, Lauvau G. Blood monocytes: distinct subsets, how they relate to dendritic cells, and their possible roles in the regulation of T-cell responses. Immunol Cell Biol. 2008;86:398–408. doi: 10.1038/icb.2008.19. [DOI] [PubMed] [Google Scholar]
- 3.Randolph GJ. Emigration of monocyte-derived cells to lymph nodes during resolution of inflammation and its failure in atherosclerosis. Curr Opin Lipidol. 2008;19:462–468. doi: 10.1097/MOL.0b013e32830d5f09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bajtay Z, Csomor E, Sandor N, Erdei A. Expression and role of Fc- and complement-receptors on human dendritic cells. Immunol Lett. 2006;104:46–52. doi: 10.1016/j.imlet.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 5.Helmy KY, Katschke KJ, Jr, Gorgani NN, Kljavin NM, Elliott JM, Diehl L, Scales SJ, Ghilardi N, van Lookeren Campagne M. CRIg: a macrophage complement receptor required for phagocytosis of circulating pathogens. Cell. 2006;124:915–927. doi: 10.1016/j.cell.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 6.Eidsmo L, Allan R, Caminschi I, van Rooijen N, Heath WR, Carbone FR. Differential migration of epidermal and dermal dendritic cells during skin infection. J Immunol. 2009;182:3165–3172. doi: 10.4049/jimmunol.0802950. [DOI] [PubMed] [Google Scholar]
- 7.Didierlaurent AM, Morel S, Lockman L, Giannini SL, Bisteau M, Carlsen H, Kielland A, Vosters O, Vanderheyde N, Schiavetti F, et al. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol. 2009;183:6186–6197. doi: 10.4049/jimmunol.0901474. [DOI] [PubMed] [Google Scholar]
- 8.Racoosin EL, Swanson JA. M-CSF-induced macropinocytosis increases solute endocytosis but not receptor-mediated endocytosis in mouse macrophages. J Cell Sci. 1992;102:867–880. doi: 10.1242/jcs.102.4.867. [DOI] [PubMed] [Google Scholar]
- 9.Norbury CC. Drinking a lot is good for dendritic cells. Immunology. 2006;117:443–451. doi: 10.1111/j.1365-2567.2006.02335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amyere M, Payrastre B, Krause U, Van Der Smissen P, Veithen A, Courtoy PJ. Constitutive macropinocytosis in oncogene-transformed fibroblasts depends on sequential permanent activation of phosphoinositide 3-kinase and phospholipase C. Mol Biol Cell. 2000;11:3453–3467. doi: 10.1091/mbc.11.10.3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liew FY, Patel M, Xu D. Toll-like receptor 2 signalling and inflammation. Ann Rheum Dis. 2005;64:104–105. doi: 10.1136/ard.2005.042515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamprou I, Tsakas S, Theodorou GL, Karakantza M, Lampropoulou M, Marmaras VJ. Uptake of LPS/E. coli/latex beads via distinct signalling pathways in medfly hemocytes: the role of MAP kinases activation and protein secretion. Biochim Biophys Acta. 2005;1744:1–10. doi: 10.1016/j.bbamcr.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 13.Foukas LC, Katsoulas HL, Paraskevopoulou N, Metheniti A, Lambropoulou M, Marmaras VJ. Phagocytosis of Escherichia coli by insect hemocytes requires both activation of the Ras/mitogen-activated protein kinase signal transduction pathway for attachment and beta3 integrin for internalization. J Biol Chem. 1998;273:14813–14818. doi: 10.1074/jbc.273.24.14813. [DOI] [PubMed] [Google Scholar]
- 14.Mizutani T, Kobayashi M, Eshita Y, Shirato K, Kimura T, Ako Y, Miyoshi H, Takasaki T, Kurane I, Kariwa H, et al. Involvement of the JNK-like protein of the Aedes albopictus mosquito cell line, C6/36, in phagocytosis, endocytosis and infection of West Nile virus. Insect Mol Biol. 2003;12:491–499. doi: 10.1046/j.1365-2583.2003.00435.x. [DOI] [PubMed] [Google Scholar]
- 15.Ulevitch RJ, Tobias PS. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 16.Fukao T, Terauchi Y, Kadowaki T, Koyasu S. Role of phosphoinositide 3-kinase signaling in mast cells: new insights from knockout mouse studies. J Mol Med. 2003;81:524–535. doi: 10.1007/s00109-003-0475-2. [DOI] [PubMed] [Google Scholar]
- 17.Fukao T, Tanabe M, Terauchi Y, Ota T, Matsuda S, Asano T, Kadowaki T, Takeuchi T, Koyasu S. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat Immunol. 2002;3:875–881. doi: 10.1038/ni825. [DOI] [PubMed] [Google Scholar]
- 18.Guha M, Mackman N. The phosphatidylinositol 3-kinase-Akt pathway limits lipopolysaccharide activation of signaling pathways and expression of inflammatory mediators in human monocytic cells. J Biol Chem. 2002;277:32124–32132. doi: 10.1074/jbc.M203298200. [DOI] [PubMed] [Google Scholar]
- 19.Hawlisch H, Belkaid Y, Baelder R, Hildeman D, Gerard C, Kohl J. C5a negatively regulates toll-like receptor 4-induced immune responses. Immunity. 2005;22:415–426. doi: 10.1016/j.immuni.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Molnarfi N, Gruaz L, Dayer JM, Burger D. Opposite regulation of IL-1beta and secreted IL-1 receptor antagonist production by phosphatidylinositide-3 kinases in human monocytes activated by lipopolysaccharides or contact with T cells. J Immunol. 2007;178:446–454. doi: 10.4049/jimmunol.178.1.446. [DOI] [PubMed] [Google Scholar]
- 21.Karsi A, Howe K, Kirkpatrick TB, Wills R, Bailey RH, Lawrence ML. Development of bioluminescent Salmonella strains for use in food safety. BMC Microbiol. 2008;8:10. doi: 10.1186/1471-2180-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones BD, Falkow S. Salmonellosis. host immune responses and bacterial virulence determinants. Annu Rev Immunol. 1996;14:533–561. doi: 10.1146/annurev.immunol.14.1.533. [DOI] [PubMed] [Google Scholar]
- 23.Guiney DG. The role of host cell death in Salmonella infections. Curr Top Microbiol Immunol. 2005;289:131–150. doi: 10.1007/3-540-27320-4_6. [DOI] [PubMed] [Google Scholar]
- 24.Hazeki K, Nigorikawa K, Hazeki O. Role of phosphoinositide 3-kinase in innate immunity. Biol Pharm Bull. 2007;30:1617–1623. doi: 10.1248/bpb.30.1617. [DOI] [PubMed] [Google Scholar]
- 25.Cremer TJ, Shah P, Cormet-Boyaka E, Valvano MA, Butchar JP, Tridandapani S. Akt-Mediated Proinflammatory Response of Mononuclear Phagocytes Infected with Burkholderia cenocepacia Occurs by a Novel GSK3{beta}-Dependent, I{kappa}B Kinase-Independent Mechanism. J Immunol. 187:635–643. doi: 10.4049/jimmunol.1003034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyd BL, Lee TM, Kruger EF, Pinchuk LM. Cytopathic and non-cytopathic bovine viral diarrhoea virus biotypes affect fluid phase uptake and mannose receptor-mediated endocytosis in bovine monocytes. Vet Immunol Immunopathol. 2004;102:53–65. doi: 10.1016/j.vetimm.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Araki N, Johnson MT, Swanson JA. A role for phosphoinositide 3-kinase in the completion of macropinocytosis and phagocytosis by macrophages. J Cell Biol. 1996;135:1249–1260. doi: 10.1083/jcb.135.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Araki N, Hatae T, Furukawa A, Swanson JA. Phosphoinositide-3-kinase-independent contractile activities associated with Fcgamma-receptor-mediated phagocytosis and macropinocytosis in macrophages. J Cell Sci. 2003;116:247–257. doi: 10.1242/jcs.00235. [DOI] [PubMed] [Google Scholar]
- 29.West MA, Prescott AR, Eskelinen EL, Ridley AJ, Watts C. Rac is required for constitutive macropinocytosis by dendritic cells but does not control its downregulation. Curr Biol. 2000;10:839–848. doi: 10.1016/s0960-9822(00)00595-9. [DOI] [PubMed] [Google Scholar]
- 30.Yano H, Nakanishi S, Kimura K, Hanai N, Saitoh Y, Fukui Y, Nonomura Y, Matsuda Y. Inhibition of histamine secretion by wortmannin through the blockade of phosphatidylinositol 3-kinase in RBL-2H3 cells. J Biol Chem. 1993;268:25846–25856. [PubMed] [Google Scholar]
- 31.Arbibe L, Mira JP, Teusch N, Kline L, Guha M, Mackman N, Godowski PJ, Ulevitch RJ, Knaus UG. Toll-like receptor 2-mediated NF-kappa B activation requires a Rac1-dependent pathway. Nat Immunol. 2000;1:533–540. doi: 10.1038/82797. [DOI] [PubMed] [Google Scholar]
- 32.Baek KH, Ha SJ, Sung YC. A novel function of phosphorothioate oligodeoxynucleotides as chemoattractants for primary macrophages. J Immunol. 2001;167:2847–2854. doi: 10.4049/jimmunol.167.5.2847. [DOI] [PubMed] [Google Scholar]
- 33.Werling D, Hope JC, Chaplin P, Collins RA, Taylor G, Howard CJ. Involvement of caveolae in the uptake of respiratory syncytial virus antigen by dendritic cells. J Leukoc Biol. 1999;66:50–58. doi: 10.1002/jlb.66.1.50. [DOI] [PubMed] [Google Scholar]
- 34.Pinchuk LM, Boyd BL, Kruger EF, Roditi I, Furger A. Bovine dendritic cells generated from monocytes and bone marrow progenitors regulate immunoglobulin production in peripheral blood B cells. Comp Immunol Microbiol Infect Dis. 2003;26:233–249. doi: 10.1016/S0147-9571(02)00061-9. [DOI] [PubMed] [Google Scholar]
- 35.Amyere M, Mettlen M, Van Der Smissen P, Platek A, Payrastre B, Veithen A, Courtoy PJ. Origin, originality, functions, subversions and molecular signalling of macropinocytosis. Int J Med Microbiol. 2002;291:487–494. doi: 10.1078/1438-4221-00157. [DOI] [PubMed] [Google Scholar]
- 36.Uto T, Akagi T, Toyama M, Nishi Y, Shima F, Akashi M, Baba M. Comparative activity of biodegradable nanoparticles with aluminum adjuvants: Antigen uptake by dendritic cells and induction of immune response in mice. Immunol Lett. 2011;140:36. doi: 10.1016/j.imlet.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Arpaia NGJ, Lau L, Sivick KE, McLaughlin LM, Jones MB, Dracheva T, Peterson SN, Monack DM, Barton GM. TLR Signaling Is Required for Salmonella typhimurium Virulence. Cell. 2011;144:675–88. doi: 10.1016/j.cell.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ojaniemi M, Glumoff V, Harju K, Liljeroos M, Vuori K, Hallman M. Phosphatidylinositol 3-kinase is involved in Toll-like receptor 4-mediated cytokine expression in mouse macrophages. Eur J Immunol. 2003;33:597–605. doi: 10.1002/eji.200323376. [DOI] [PubMed] [Google Scholar]
- 39.Nakanishi S, Yano H, Matsuda Y. Novel functions of phosphatidylinositol 3-kinase in terminally differentiated cells. Cell Signal. 1995;7:545–557. doi: 10.1016/0898-6568(95)00033-l. [DOI] [PubMed] [Google Scholar]
- 40.Herrera-Velit P, Knutson KL, Reiner NE. Phosphatidylinositol 3-kinase-dependent activation of protein kinase C-zeta in bacterial lipopolysaccharide-treated human monocytes. J Biol Chem. 1997;272:16445–16452. doi: 10.1074/jbc.272.26.16445. [DOI] [PubMed] [Google Scholar]
- 41.Taylor PR, Gordon S. Monocyte heterogeneity and innate immunity. Immunity. 2003;19:2–4. doi: 10.1016/s1074-7613(03)00178-x. [DOI] [PubMed] [Google Scholar]
- 42.Mercer J, Helenius A. Virus entry by macropinocytosis. Nat Cell Biol. 2009;11:510–520. doi: 10.1038/ncb0509-510. [DOI] [PubMed] [Google Scholar]
- 43.Amstutz B, Gastaldelli M, Kalin S, Imelli N, Boucke K, Wandeler E, Mercer J, Hemmi S, Greber UF. Subversion of CtBP1-controlled macropinocytosis by human adenovirus serotype 3. EMBO J. 2008;27:956–969. doi: 10.1038/emboj.2008.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sirena D, Lilienfeld B, Eisenhut M, Kalin S, Boucke K, Beerli RR, Vogt L, Ruedl C, Bachmann MF, Greber UF, et al. The human membrane cofactor CD46 is a receptor for species B adenovirus serotype 3. J Virol. 2004;78:4454–4462. doi: 10.1128/JVI.78.9.4454-4462.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wickham TJ, Mathias P, Cheresh DA, Nemerow GR. Integrins alpha v beta 3 and alpha v beta 5 promote adenovirus internalization but not virus attachment. Cell. 1993;73:309–319. doi: 10.1016/0092-8674(93)90231-e. [DOI] [PubMed] [Google Scholar]
- 46.Karjalainen M, Kakkonen E, Upla P, Paloranta H, Kankaanpaa P, Liberali P, Renkema GH, Hyypia T, Heino J, Marjomaki V. A Raft-derived, Pak1-regulated entry participates in alpha2beta1 integrin-dependent sorting to caveosomes. Mol Biol Cell. 2008;19:2857–2869. doi: 10.1091/mbc.E07-10-1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meier O, Greber UF. Adenovirus endocytosis. J Gene Med. 2003;5:451–462. doi: 10.1002/jgm.409. [DOI] [PubMed] [Google Scholar]
- 48.Lee JY, Bowden DS. Rubella virus replication and links to teratogenicity. Clin Microbiol Rev. 2000;13:571–587. doi: 10.1128/cmr.13.4.571-587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karsi A, Lawrence ML. Broad host range fluorescence and bioluminescence expression vectors for Gram-negative bacteria. Plasmid. 2007;57:286–295. doi: 10.1016/j.plasmid.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 50.Collins FM. Growth of Pasteurella multocida in vaccinated and normal mice. Infect Immun. 1973;8:868–875. doi: 10.1128/iai.8.6.868-875.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]