Figure 2.
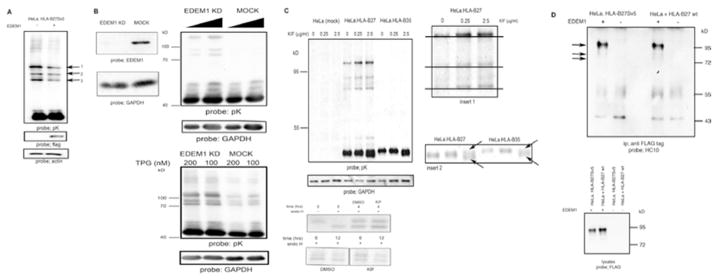
EDEM1 modulates HLA-B27 dimers; (A) Over expression of EDEM1 can enhance HLA-B27 dimer degradation. HeLa-B27 were transfected with EDEM1-FLAG, lysed and immunoblotted for MHC class I heavy chain. Arrows 1, 2 and 3 indicate HLA-B27 dimers. Lysates immunoblotted with anti-FLAG demonstrate EDEM1 expression. (B) shRNA inhibition of EDEM1 expression (left panel) in HeLa-B27 cells prevents HLA-B27 dimer ERAD when compared to mock transduction with a control scrambled shRNA (middle panel). HLA-B27 dimers are resistant to ER stress induced degradation following EDEM1 knockdown (right panel). (C) Increasing concentrations of kifuesine (KIF) lead to enhanced detection of dimers as determined by immunoblotting of HeLa-B27.Sv5. Immunoblotting for GAPDH reveals equal loading. Insert 1 demonstrates that with increasing KIF concentrations the high Mw dimers exhibit small changes in Mw. Insert 2 arrows indicate that at 2.5 μg/ml KIF, the monomer of both HLA-B27:05 and –B*35:01 can be resolved as a doublet (left panel) Kifuensine does not alter the maturation rate of HLA-B27. HeLa-B27.Sv5 were radiolabeled and chased in the presence of kifuensine or vehicle control DMSO (right panel). (D) HLA-B27 ER resident dimers preferentially associate with EDEM1 (left panel). HeLa-B27.Sv5 were transfected with EDEM1-FLAG, or HeLa cells were co-transfected with HLA-B27 and EDEM1-FLAG and cell lysates were immunoprecipitated with an anti-FLAG tag antibody and immunoblotted for class I heavy chain (left panel). Immunoblotting lysates with anti-FLAG from HeLa and HeLa-B27Sv5 cells transfected with EDEM1 demonstrates equal levels of EDEM1 expression (right panel).
