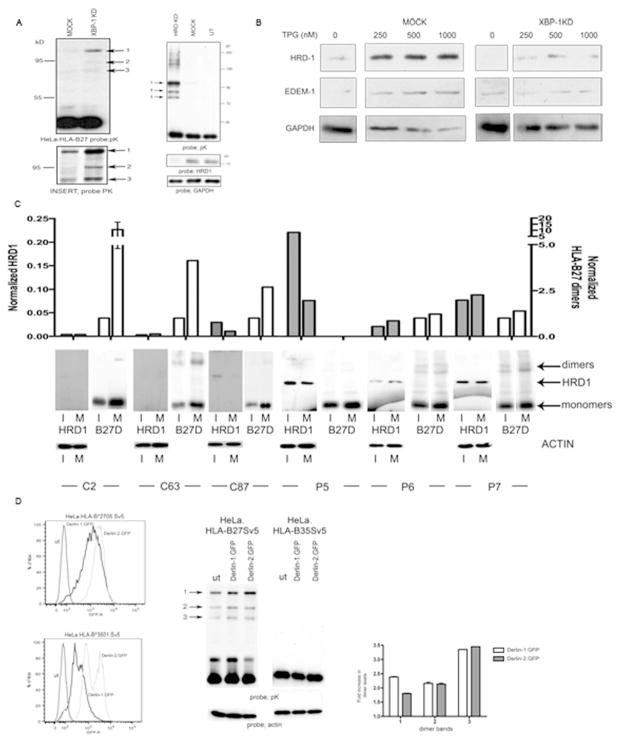
Figure 4.
XBP-1, HRD1 and Derlin1/2 participate in HLA-B27 dimer degradation. (A) Degradation of HLA-B27 is dependent on the XBP-1 pathway. shXBP1 inhibition demonstrates enhanced dimerisation of HLA-B27, with Insert showing the dimers following longer exposure (left panel). shRNA inhibition of HRD1 expression in HeLa-B27 cells prevents HLA-B27 dimer ERAD when compared to mock transduction with a control shRNA (shLUC). Immunoblotting lysates demonstrates that HRD1 has been down regulated (right panel). (B) HRD1 and EDEM1 depend on XBP-1 for ER stress induction. ShRNA inhibition of XBP-1 does not lead to induction of HRD1 and EDEM1 following ER stress induction with thapsigargin (TPG) compared to shLUC mock control cells. (C) HRD1 and HC10 reactive HLA-B27 dimer levels were quantified in both immature (I) and mature (M) dendritic cells generated from HLA-B27 patients. HRD1 levels can be elevated in dendritic cells from AS patients. Open bars represent normalised B27 dimer (D) levels and shaded bars represent normalised HRD1 levels. (D) Derlin-1 and –2 can participate in HLA-B27 dimer ERAD. Derlin-1.GFP and Derlin-2.GFP expression following retroviral infection of HeLa cells expressing HLA-B27 and HLA-B35 (left panel). HeLa-B27 cells were transduced with Derlin-1 and -2 GFP tagged proteins, which act as dominant negative inhibitors of the respective Derlin activities. Immunoblotting revealed that both Derlin-1 and –2 can modestly enhance HLA-B27 dimer formation, leading to the enhanced detection of dimers as highlighted by arrows 1, 2 and 3 (middle panel). Quantitation of the different dimer bands 1, 2 and 3 reveals a 2–3 fold increase in their detection when Derlin 1 and 2 activities are inhibited (right panel).