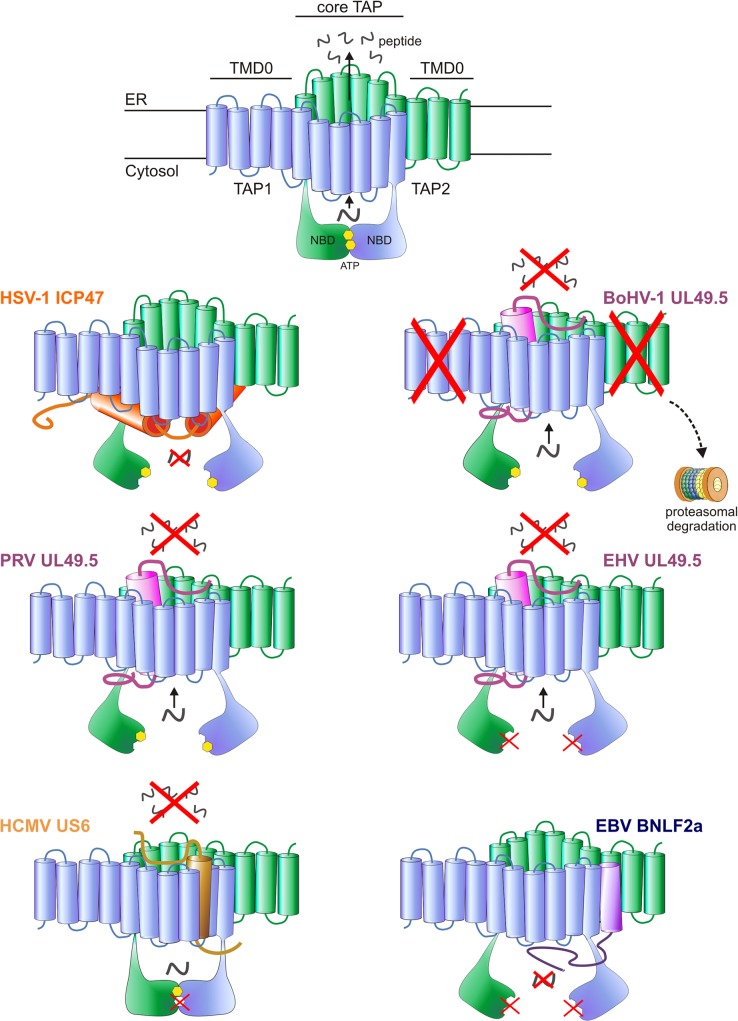
Fig 1. Interactions between herpesvirus-encoded TAP-inhibitors and their target.
Upper illustration: model of the TAP transporter, comprising the two subunits TAP1 and TAP2. Each subunit contains a transmembrane domain (TMD), encompassing 10 and 9 transmembrane (TM) helices for TAP1 and TAP2, respectively. The outer N-terminal helices of TAP1 and TAP2 (TMD0) form an autonomous binding platform for tapasin, whereas the core 6 TM helices are necessary for peptide transport. A peptide-binding domain is located within the cytosolic extensions of the TM helices. In addition, TAP1 and TAP2 contain a nucleotide-binding domain (NBD) in the cytosol, which harbors two ATP-binding sites. Lower illustrations: schematic representations of the interaction between the viral proteins and TAP. The sites where TAP is affected are indicated. HSV-1 ICP47 prevents peptide transport by physically obstructing the peptide-binding site. PRV, BoHV-1 and EHV (EHV-1 and EHV-4) UL49.5 leave the transporter in a transformation-incompetent conformation, thereby preventing the structural changes that are needed to translocate peptides over the ER membrane. BoHV-1 UL49.5 is known to interact with a region within the core domain of TAP, comprising the C-terminal 6 TM domains of both TAP1 and TAP2 [132]. BoHV-1 UL49.5 induces the degradation of both TAP subunits, and EHV UL49.5 prevents ATP binding to TAP. HCMV US6 blocks TAP by inducing conformational changes that result in diminished ATP binding to TAP1. The protein interacts with TM domains 7–10 of TAP 1 and TM 1–4 of TAP2 [95]. EBV BNLF2a inhibits peptide transport by interfering with both peptide and ATP binding to TAP.