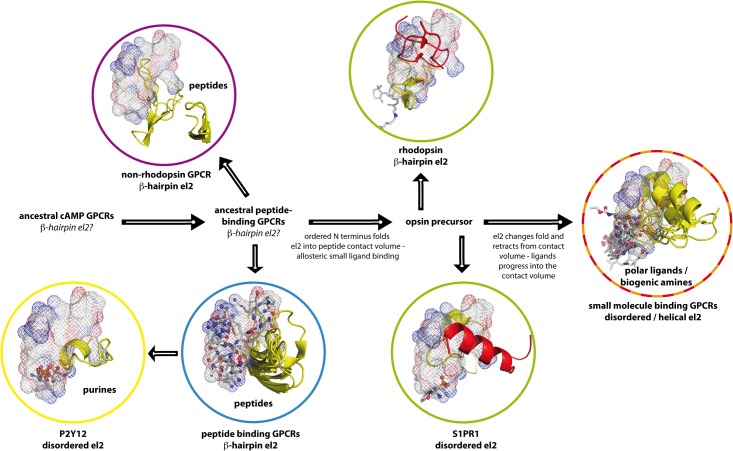
Fig 4. Development of peptide and small molecule binding GPCRs by protein self-interaction with el2.
Peptide ligand binding volume in rhodopsin-like GPCRs (see Fig 3) as mesh, small organic ligands (see Fig 3) in grey sticks with polar oxygen / nitrogen atoms as spheres, el2 as yellow cartoon, N termini as red cartoon. In all known non-rhodopsin GPCR structures (purple circle) [23–25,28], el2 forms a β–hairpin structure, which surrounds the peptide binding volume. In rhodopsin-like peptide receptor structures with bound peptide ligand (blue circle) [9,18], el2 forms a β-hairpin, which flanks this domain, too. This common arrangement suggests that during evolution, both rhodopsin-like and non-rhodopsin peptide binders kept common ancestral peptide ligand binding features. Furthermore, both classes kept a common position and shape of el2. The length of the rhodopsin el2 is in good agreement with the one observed in peptide-binding GPCRs. In rhodopsin (green circle, top), el2 goes right through the common peptide / small molecule ligand volume, and is held there by steric constraints from the N terminus (see Fig 3B). Retinal binds underneath and outside of this contact domain at a position close to the protein center. In the sphingosine receptor (green circle, bottom), el2 is disordered and has contracted in comparison to rhodopsin, while still being in contact with the N terminus (compare Fig 3C). The ligand has advanced into the common ligand domain, performing contacts there. We therefore assume that an opsin precursor forms the link between peptide-binding GPCRs and small molecule-binding GPCRs. In small molecule-binding GPCRs (red / orange circle), el2 is disordered or helical and has retracted from the peptide-binding domain. Small molecule ligands bind in the peptide contact volume at the position of el2 in rhodopsin, and below it. It seems that the ancestral rhodopsin-like GPCRs initially possessed an el2 in the form of a β-hairpin. Peptide binders retained el2 in this form. Small molecule ligands for GPCRs seem to have then developed as allosteric binders via an opsin ancestor as key intermediate, with el2 substituting a bound peptide ligand. Along the proposed pathway, small molecule ligands then seem to have substituted el2, who retracted from the binding site and lost the β-hairpin conformation. Purine receptors seem to have undergone a similar development.