Figure 2. Mechanism for ‘fusing’ the C-spine.
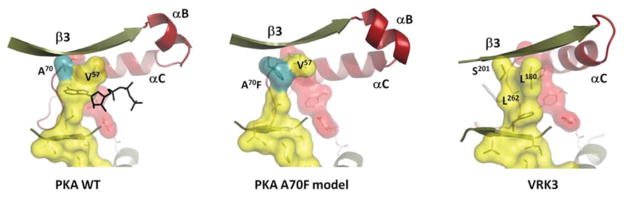
Two elements of the endogenous C-spine are fused by the adenine ring of ATP (left). We predict that the phenylalanine side chain in the A70F mutant will fill in the adenine-binding pocket (centre). In VRK3, several residues (Leu180, Ser201 and Leu262) fill in the adenine-binding pocket making it unable to bind ATP. Leu180 and Ser201 are equivalent to Val57 and Ala70 respectively in PKA, whereas Leu262 corresponds to Val123 from the hinge.
