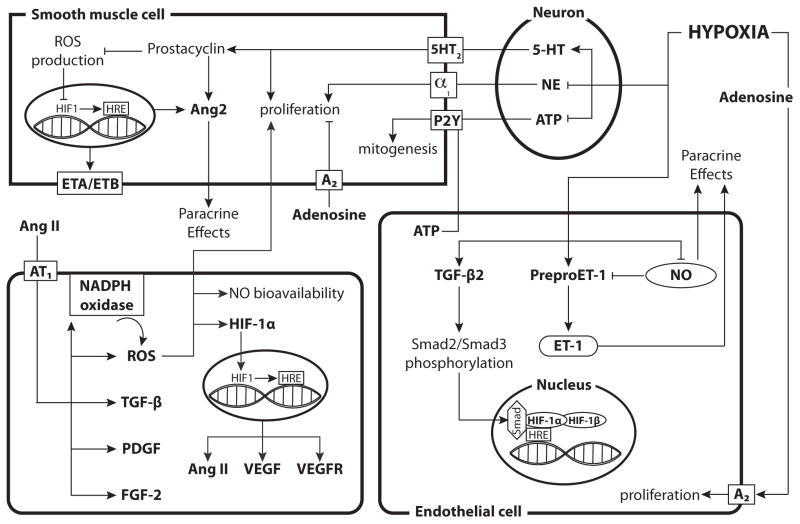
Fig. 4. Hypoxia has heterogeneous effects on Receptor Tyrosine Kinase-independent vasotrophic factor signaling across various cell types.
In perivascular nerves, hypoxia inhibits the synthesis and decreases the content of NE while enhancing serotonin (5-HT) synthesis. Elevated 5-HT levels can then induce proliferation of smooth muscle cells and increase prostacyclin levels, which inhibits ROS production and increase Ang2 production. Adenosine can activate A2 receptors and inhibit proliferation while ATP enhances mitogenesis in SMCs but can also increase endothelial cell proliferation. Hypoxia enhances the expression of TGF-β, preproET-1 and ET-1 while inhibiting NO synthesis in endothelial cells. Hypoxia also enhances expression of both ET receptors in smooth muscle, thereby enhancing the effects of ET-1. Increased TGF-β2 levels enhance Smad2/Smad3 phosphorylation, which can then act as a coactivator for HIF-1. Angiotensin II activates AT1, which leads to an increase in FGF-2, PDGF, TGF-β, and NADPH oxidase. Increased NADPH oxidase leads to enhanced ROS production, which can inhibit NO bioavailability and induce hypertrophy and hyperplasia of smooth muscle cells. ROS can also increase the gene expression of HIF-1α and stabilize the HIF-1α protein. The HIF-1 complex then enters the nucleus, binds HREs, and results in increased transcription of VEGF, VEGF receptors, and Ang II. The diagram includes separate depictions of mechanisms in neurons, smooth muscle cells, and endothelial cells. For reference, a generic (parenchymal) cell is depicted in the lower left corner.