Fig 4. Comparison of initial and final peptide-bond planes for 25608.37. A, C, E, G, I, K and M.
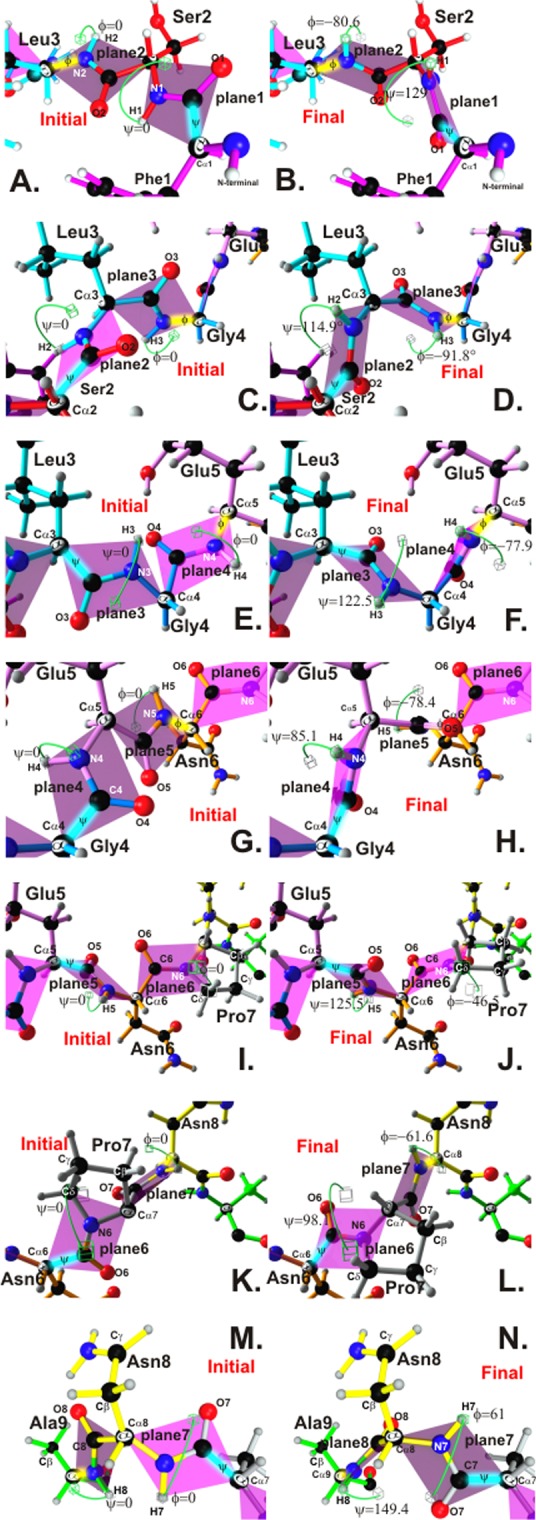
Green cubes indicate initial theoretical positions for 25068.37 regarding PBR residues forming Φ and ψ angles on planes 1 to 9. B., D., F., H., J., L. and N. Final position of these angles reaching the lowest energy to avoid topochemical steric clashes, as measured for the lowest energy conformer, based on 3D structure obtained by 1H-NMR. Most angles were close to -93° ± 25° (Φ) and 134° ± 15° (Ψ), similar to PPIIL. Minimisation values for atom clashes as determined by Ramachandran plot. HLA-DRβ1* classification according to experimental binding to purified molecules, binding motifs and binding registers is shown at the top on the right-hand side.
