Abstract
Objective
Cigarette smoking is highly prevalent among people with bipolar disorder or schizophrenia. Few studies have examined whether smoking history is associated with adaptive functioning among individuals diagnosed with these serious mental illnesses.
Method
In a large relatively homogenous cohort of patients with either bipolar disorder (n=363) or schizophrenia (n=400), we investigated the association between cigarette smoking status, intensity, and cumulative exposure and performance on a comprehensive battery of neurocognitive, functional capacity, informant-rated functional measures. The associations were adjusted for variation in sociodemographic indicators, psychopathologic symptoms, and substance use.
Results
There was an average of 12 pack years of smoking across the sample. People with schizophrenia reported double the rate of current smoking compared to patients with bipolar disorder. Adjusting for demographic covariates, current smokers had worse composite cognitive functioning and poorer functional outcome than past or never smokers. There were no significant differences between never and past smokers, and these effects were evident in both bipolar disorder and schizophrenia.
Conclusion
Current smokers with either schizophrenia or bipolar disorder evidence worse cognitive and adaptive functioning functional outcome, even when demographic covariates are considered.
Significant Outcomes
Patients with schizophrenia had double the rate of smoking compared to patients with bipolar disorder
Current smoking was negatively associated with cognitive functioning, functional capacity, and informant reported functional outcomes in both patients with schizophrenia and bipolar disorder, after adjusting for sociodemographic covariates
Limitations
The study was cross-sectional and so causal associations cannot be inferred
Tobacco use was assessed with a self-report instrument
The sample was relatively homogenous and high function and may not generalize to ethnically diverse or more symptomatic samples
Keywords: Cognition, Neuropsychology, Schizophrenia, Psychosis, Bipolar Disorder, Nicotine
INTRODUCTION
Smoking continues to be highly prevalent among patients with serious mental illnesses. Approximately 60% of adults with schizophrenia are current cigarette smokers (1) and 45% of patients with bipolar disorder are smokers (1); these rates are about 2–3 times the rate of smoking in the U.S. adult population (2). There remains considerable controversy about the impact of cigarette smoking on cognitive and adaptive functioning in these two most disabling serious mental disorders (SMI)(3).
On one hand, higher rates of smoking are attributed to the greatly elevated rates of cardiovascular morbidity and mortality among patients with SMI, which occurs approximately 10–25 years earlier than that in healthy comparators (4). In population-based samples of adults, exposure to smoking during mid-life predicts greater cognitive impairment in later life (5, 6) Although there is no clear understanding of how cigarette smoking may negatively impact cognition over the lifespan, it has been suggested that smoking may hasten progression in white matter deterioration and increase inflammation (7). On the other hand, there is some data from translational models, experimental studies, and clinical studies indicating that nicotine may be associated with better short-term cognitive performance in schizophrenia (8–12)(8) and in a smaller number of studies in bipolar disorder (9, 10). Several clinical studies have shown comparatively better performance on cognitive tests, particularly attentional tasks, among current smokers compared to non-smokers with schizophrenia (11). Predominant theories about the mechanism underlying the potential positive influence of nicotine on cognition in schizophrenia include enhanced sensory auditory gating and the involvement of nicotinic receptors in working memory (12, 13). On balance, other studies have indicated that nicotine has no detectable effects (14, 15) or negative effects on cognitive task performance in schizophrenia (16) (17).
A significant and frequent limitation with prior research employing clinical samples to assess the association between smoking history and cognitive function in SMI is that most samples have been modestly sized, and thus have had limited power to adjust for the impact of sociodemographic and clinical covariates or potential lifespan “dose” effects of smoking (18). The population distribution of smoking is highly confounded with educational attainment, socioeconomic status, and comorbid abuse of alcohol or other illicit substances. Therefore, sociodemographically heterogeneous samples that are of modest size are often poorly equipped to tease apart the relative impact of smoking on cognitive performance in the context of associated covariates. Moreover, given the strong link between cognition and functional status in both schizophrenia and bipolar disorder (19, 20), it would stand to reason that smoking might also associate with scores on performance-based functional skills tasks and ratings of functional outcome. However, no studies to our knowledge have examined the association of smoking history with functional capacity or everyday functional outcomes in bipolar disorder or schizophrenia.
AIMS OF THE STUDY
To help shed light on the impact of smoking history on cognitive performance and functional capacity in schizophrenia and bipolar disorder, we assessed the cross-sectional association of various indicators of self-reported smoking history (e.g., current status, smoking intensity in pack years) with performance on a neuropsychological and functional tests, making statistical adjustments for demographic and clinical covariates. We hypothesized, based on clinical and translational literature in SMI samples, that current and former smokers would perform better than never smokers on cognitive testing, functional capacity, and functional outcome, after adjusting for covariates.
MATERIAL AND METHODS
Sample
All participants were originally enrolled in a parent study focusing on the genetics of schizophrenia and bipolar disorder [(the complete methodology of the parent study is described elsewhere (16–18)]. Participation was restricted to persons of full or mixed Ashkenazi Jewish descent, determined on the basis of ancestry of four grandparents. The purpose of this restriction was to leverage potential founder effects in this population for genetic studies (21). Participants were recruited via advertisements, websites, and publications marketed toward people of Jewish descent. Participants were enrolled in the parent study between 1996 and 2006, completing an in-person clinical interview (the Diagnostic Interview for Genetics Studies; DIGS) (22), blood drawing, and family history interview (Family Interview for Genetic Studies). Participants were interviewed in their place of residence by PhD-level clinical psychologists.
Between 2007 and 2012, subjects diagnosed with bipolar I, schizophrenia or schizoaffective disorder in the parent study were re-contacted to participate in a follow-on study in which participants were administered a battery of neurocognitive and functional capacity/outcome measures as well as measures pertaining to smoking history (smoking status was not assessed in the earlier assessments). Participants were once again seen in their place of residence for administration of the follow-up study measures. All participants signed written informed consent to participate both the parent and follow-on studies, and both studies were approved by the Johns Hopkins Medicine Institutional Review Board.
Diagnoses
Participants were diagnosed at the time of their enrollment in the parent study. Diagnoses were based on an independent review of all available information (i.e., the in-person clinical interview, information reported by informants, treatment records) by two members of a diagnostic committee composed of psychiatrists and psychologists. Diagnoses were made according to DSM-IV criteria, and required consensus between the two independent reviewers. Participants were categorized by diagnosis into bipolar disorder (all were bipolar disorder, type 1) and schizophrenia/schizoaffective disorder. Although diagnoses were not reconfirmed at the time of the other variables, we note that diagnoses registered by doctoral level examiners tend to be stable over time(23).
Independent Variable
Smoking status and history
Smoking histories were self-reported and derived from the UPSIT(24), gathered at the same time as neuropsychological and functional test data was obtained. We extracted the following variables from the measure for use in analyses: 1) Lifetime smoking pattern as current, past, and never smoker; 2) the average number of cigarettes smoked per day, 3) the age at onset of smoking, and 4) time in years since the age of smoking cessation among ex-smokers. We calculated pack years as the duration of smoking in years multiplied by the number of cigarettes smoked per day, divided by twenty. A number of studies have found that, in population-based samples, self-reported smoking status measures are valid in the extent that they are highly consistent with objective biomarkers such as urinary cotinine or CO levels (25, 26)
Dependent Variables
Neurocognitive Ability
We calculated a Neurocognitive Composite Score derived from a set of commonly used neuropsychological tests addressing verbal memory (RAVLT learning) processing speed (Trail Making Test Part A, WAIS-III Digit Symbol), switching (Trail Making Test Part B), working memory (WAIS-III Letter Number Sequencing), verbal fluency (Animal Fluency), problem-solving (Wisconsin Card Sorting Test Perseverative Errors), and sustained attention (Continuous Performance Test Identical Pairs version, d-Prime). Raw scores on these 8 measures were normed based on published normative data for each of the tests. To obtain the Composite Neurocognitive Score we transformed variables to Z-scores and then obtained an average Z-score across all tests, as in multiple previously published studies. The internal consistency of the neuropsychological variables used to create the composite score was Cronbach’s alpha = 0.81 in bipolar disorder and 0.83 in schizophrenia. (See our previous publications on this sample for the normative basis and validity of this composite measure: e.g., Bowie et al.(20)).
Functional Capacity
Participants were administered the brief version of the UCSD Performance-based Skills Assessment (UPSA-B)(27). The UPSA-B assesses the participant’s capacity to perform tasks similar to those encountered in daily life. Two domains are assessed on the UPSA-B: 1) Financial skills, in which participants are required to count change, make change from an item purchased at a store, and write a check for a utility bill, and 2) Communication skills, in which participants are asked to demonstrate how to use a telephone to dial emergency services, call information to ask for a telephone number, and call a physician to reschedule a medical appointment. A summary score is then calculated by summing the two domain scores (range = 0–100), with higher scores indicating better functional capacity.
Informant-Rated Functional Outcome
Participants’ everyday functioning was assessed using the Specific Level of Functioning (SLOF) scale. For this scale, an informant rates the participant’s ability to perform 43 specific functional tasks encompassing 6 domains: a) physical functioning, b) personal care skills, c) interpersonal relationships, d) social acceptability, e) activities, and f) work skills. Ratings are made on a 5-point Likert scale indicating the level of assistance the participant needs to perform the task, with higher scores indicating better functioning. Ratings were provided by family members, board-and-care managers or a caseworker familiar with the patient’s level of functioning. The SLOF has excellent reliability and validity(28) and is among the most valid measures of functioning in patients with schizophrenia(29–31). The physical functioning, personal care skills, and social acceptability scales assess basic (lower order) functioning, whereas the daily activities, work skills, and interpersonal relationships subscales assess higher order functional activities. Because of ceiling effects on the lower order activity markers, we calculated a total functioning score using the sum of the daily activities, work skills, and interpersonal relationships scales.
Potential Covariates
Demographics and Health Covariates
Demographic factors that were included as potential covariates were age, sex, educational attainment, and marital status. We also included Body Mass Index as a potential covariate due to its prior association with cognitive functioning in this sample (32) and co-occurrence of obesity with cigarette smoking (33). A final covariate was residential status. Participants were interviewed by clinical examiners regarding their current living arrangement and were consequently coded by assessors into one of four residential statuses: a) head of household, independent (i.e., live alone or with others and have primary or co-equal financial and/or logistical responsibility for the household), b) head of household, semi-independent (bears only partial and not co-equal financial and/or logistical responsibility for the household, c) not head of household, but in community (i.e., living in a group home, or as a dependent in the home of their parents or children, etc.), and d) residential treatment facility (i.e., have a degree of community exposure but require residence in a treatment environment). For the purposes of our primary analyses, participants who were heads of household and either independent or semi-independent were classified as “independent”, and those who were not heads of household or were residing in a treatment facility were classified as “not independent.”
Substance abuse
With regard to substance use, participants were asked about current use of alcohol and other substances (i.e., cocaine, amphetamine, marijuana, hallucinogens, and non-prescribed opiates or sedatives). Alcohol use intensity was assessed by use of the Khavari Alcohol Test, a self-report assessment that estimates the number and volume of alcoholic drinks the patient consumed during the past month (34). The Annual Absolute Alcohol Index (AAAI was calculated based on the scores to provide a consumption variable. We pooled the illicit substances listed above into a single binary indicator reflecting the presence of any use in the past month of any of the substances.
Affective and Psychotic Symptoms
Depressive symptoms were assessed with the self-report Beck Depression Inventory – II (BDI; (35) and psychotic symptoms with the interviewer-rated Positive and Negative Syndrome Scale (PANSS; (36). The BDI is a widely used 21-item self-report measure of depressive symptoms, with good internal consistency (Schizophrenia: Cronbach’s alpha=0.89; Bipolar disorder: Cronbach’s alpha=0.93). The PANSS was rated by doctoral-level clinicians and also evidenced acceptable internal consistency for both the Positive (Schizophrenia Cronbach alpha = 0.71, Bipolar disorder Cronbach alpha = 0.72) and Negative Syndrome Scale scores (Schizophrenia Cronbach alpha = 0.85 and Bipolar Disorder Cronbach alpha = 0.65).
Statistical Analyses
We first contrasted groups by smoking status (never, prior smoker, or current smoker) using ANOVA and Chi-square tests, and for subsequent models we included as covariates any variables that were significant at the 0.05 alpha level.. We then examined the relationship between smoking status and three outcomes: 1) Cognitive Composite, 2) UPSA Score, and 3) SLOF higher-order functioning score. Three separate models were run with the General Linear Model procedure dwith 1) an unadjusted model, 2) a demographically-adjusted model, and 3) a demographically and clinical symptom severity adjusted model. Each analysis included diagnosis as a main effect and an interaction between diagnosis and smoking status. All effects were fixed effects. Pair-wise contrasts (e.g., ex-smokers vs. current smokers) were assessed using Tukey adjustment. We calculated effect sizes for these contrasts by dividing the mean difference identified in pair-wise comparisons by the pooled standard deviation (Cohen’s d). Since pack years was a continuous variable, we conducted linear regressions examining the association between pack years of smoking, duration of smoking, and cigarettes smoked per day as independent variables (all fixed effects), entering any covariates identified as above in the first step. Regressions were conducted separately across the three outcomes as above as dependent variables. We adjusted for test-wise comparisons by dividing 0.05 by three (representing the separate analyses for Cognitive Composite, UPSA, and SLOF scores) for a study-wide alpha of 0.017.
RESULTS
Sample Characteristics
Demographic and clinical characteristics of the 363 patients with bipolar disorder and 400 patients with schizophrenia by smoking status (Never smoked, ex-smoker, current smoker) are displayed in Table 1. In the bipolar group, ex-smokers were significantly older, and current smokers completed fewer years of education. In the group with schizophrenia, never smokers were younger than ex-smokers, and ex-smokers were more likely to be women and reside independently. As with bipolar disorder, current smokers completed fewer years of education. In both bipolar disorder and schizophrenia, never smokers had less severe PANSS positive syndrome scale scores. Ex-smokers with bipolar disorder had lower PANSS negative syndrome scale scores. It is worth noting that the average symptom severity of BDI and PANSS scores were in the mild range in both of the diagnostic groups. Current and ex-smokers were more likely to endorse higher intensity alcohol use and also past month illicit drug use. Smoking history was not associated with BMI.
Table 1.
Comparison of Demographic, Clinical, and Smoking Data
| Variable | Never-smokers (BD; n=218) (SZ; n= 200) M(SD) or % |
Ex-Smokers (BD; n=101) (SZ; n=98) M(SD) or % |
Current Smokers (BD; n=44) (SZ; n=104) M(SD) or % |
F-value | p-value | Pairwise comparison C=Current N=Never E=Ex-smoker |
|---|---|---|---|---|---|---|
| Age | ||||||
| BD | 46.8 (12.9) | 52.8 (13.6) | 43.3 (10.8) | 10.9 | <0.001 | C=N<E |
| SZ | 48.8 (10.7) | 52.7 (9.5) | 50.4 (8.5) | 5.2 | <0.001 | N<E |
| Sex (% female) | ||||||
| BD | 48.6% | 56.4% | 50.0% | 1.7 | 0.427 | ------- |
| SZ | 34.0% | 45.9% | 28.8% | 6.8 | 0.033 | ------- |
| Education | ||||||
| BD | 15.0 (2.8) | 15.3 (2.1) | 14.0 (2.8) | 4.1 | 0.017 | C<N=E |
| SZ | 14.4 (2.4) | 14.7 (2.2) | 13.8 (2.2) | 4.0 | 0.018 | C<E |
| Independent living | ||||||
| BD | 84.4% | 90.1% | 88.6% | 2.1 | 0.348 | ------- |
| SZ | 64.0% | 75.5% | 58.7% | 6.7 | 0.035 | ------- |
| Age of Onset | ||||||
| BD | 18.7 (8.0) | 20.0 (8.0) | 16.2 (6.5) | 3.6 | 0.028 | C<E |
| SZ | 20.6 (6.0) | 19.3 (5.6) | 20.2 (5.2) | 1.6 | 0.200 | ------- |
| BMI | ||||||
| BD | 27.9 (6.2) | 28.4 (6.2) | 28.6 (6.1) | 0.3 | 0.708 | ------- |
| SZ | 30.4 (6.9) | 30.0 (6.4) | 29.3 (5.7) | 0.9 | 0.414 | ------- |
| Alcohol Use Index | ||||||
| BD | 1.3 (3.2) | 1.7 (3.2) | 3.8 (15.4) | 3.1 | 0.045 | ------- |
| SZ | 0.2 (0.8) | 1.1 (7.7) | 1.3 (5.5) | 2.0 | 0.131 | ------- |
| Illicit Drug Use in Past Month | ||||||
| BD | 6.0% | 11.9% | 29.5% | 22.0 | <0.001 | |
| SZ | 1.5% | 5.1% | 8.7% | 8.9 | 0.012 | |
| Depressive symptoms (BDI) | ||||||
| BD | 9.1 (10.0) | 9.9 (10.3) | 11.0 (10.3) | 0.8 | 0.471 | ------- |
| SZ | 10.1 (8.0) | 12.8 (10.4) | 12.4 (10.3) | 3.8 | 0.023 | ------- |
| PANSS Positive | ||||||
| BD | 10.4 (3.8) | 9.9 (3.1) | 12.0 (4.9) | 4.8 | 0.009 | N=E<C |
| SZ | 14.6 (5.9) | 15.3 (6.6) | 17.2 (6.7) | 5.9 | 0.003 | N<C |
| PANSS Negative | ||||||
| BD | 9.4 (3.2) | 8.5 (2.0) | 10.0 (3.6) | 4.5 | 0.012 | E<C |
| SZ | 15.7 (7.3) | 14.1 (6.0) | 15.9 (8.1) | 1.9 | 0.145 | ------- |
| PANSS Excitement | ||||||
| BD | 6.5 (3.2) | 6.0 (2.2) | 7.5 (4.2) | 3.6 | 0.027 | ------- |
| SZ | 6.0 (2.8) | 5.7 (2.5) | 6.4 (3.3) | 1.8 | 0.175 | ------- |
| Duration of Smoking | ||||||
| BD | ------- | 15.9 (11.3) | 23.7 (12.5) | 13.5 | <0.001 | ------- |
| SZ | ------- | 17.3 (12.6) | 30.8 (11.4) | 62.5 | <0.001 | ------- |
| Cigarettes Per Day | ||||||
| BD | ------- | 18.4 (12.9) | 19.9 (12.3) | 0.4 | 0.516 | ------- |
| SZ | ------- | 26.5 (17.5) | 24.8 (13.3) | 0.6 | 0.430 | ------- |
| Pack-Years | ||||||
| BD | ------- | 18.2 (23.8) | 26.0 (24.1) | 3.2 | 0.074 | ------- |
| SZ | ------- | 25.3 (26.4) | 38.9 (25.4) | 13.6 | <0.001 | ------- |
| Age Started Smoking | ||||||
| BD | ------- | 18.9 (5.7) | 19.6 (8.7) | 0.3 | 0.565 | ------- |
| SZ | ------- | 19.7 (7.4) | 19.6 (7.2) | 0.1 | 0.875 | ------- |
BD: Bipolar Disorder; SZ: Schizophrenia; BMI: Body Mass Index; PANSS: Positive and Negative Syndrome Scale; BDI: Beck Depression Inventory
Smoking Variables and Associations
The rate of current smoking in schizophrenia (25.9%) was double that of the group with bipolar disorder (12.1%) while the rate of ex-smokers in the two diagnostic groups was comparable (27.4% in bipolar disorder vs. 24% in schizophrenia). Compared to prior studies, the rate of current smoking in this sample was considerably lower. As seen in Table 1, among smokers, the mean number of pack years of smoking in the schizophrenic subjects was twice that of the subjects diagnosed with bipolar disorder. Similarly, the number of cigarettes smoked per day was significantly higher in the schizophrenia group.
Association between Smoking History and Cognitive Functioning
In an unadjusted model, smoking history was significantly associated with cognitive functioning (F (2, 757)=6.8, p<0.001), with no interaction effect evident with diagnosis. Post-hoc tests indicated that current smokers evidenced worse cognitive functioning than ex- or never smokers, with no significant difference in functioning between ex- and never smokers. The effect size (Cohen’s D) for the difference between ex- and never smokers was d=0.09, where as the effect size for the difference between current smokers and ex- and never smokers was d=0.41. The effect of smoking history remained significant after adjusting for significant demographic covariates (i.e., age, sex, education, residential independence, F (2, 740)=4.1, p=0.011), again with no significant smoking by diagnosis interaction effect. After additionally adjusting for symptoms (PANSS Positive and Negative Syndrome Scales) and past month use of illicit substances and alcohol use intensity, the effect of smoking history on cognitive functioning was no longer significant at the alpha level of 0.017 (F, 2,734)=3.1, p=0.045). Post-hoc tests indicated only one significant pairwise difference with current smokers showing poorer performance than never smokers. Exploratory analyses of the individual cognitive tests and smoking status revealed that only test, the WAIS Digit Symbol, demonstrated a statistically significant association.
Among smokers, pack years were negatively associated with cognitive functioning in the hierarchical regression analysis, adjusting for demographic variables, substance abuse/alcohol use and symptoms (r2 change = 0.027, F change 1, 301 = 14.1, p<0.001). Similarly, the number of cigarettes smoked per day was negatively associated with cognitive functioning (r2 change = 0.030, F change 1, 317, p<0.001). Investigation of exponential associations (i.e., quadratic, cubic) did not provide better fits than did linear modeling. Each of these relationships persisted when adding demographic and symptom covariates and did not significantly differ by diagnosis. Among ex-smokers, years since stopped smoking was not associated with the Cognitive Composite score.
Association between Smoking History and Adaptive Functioning
As with cognitive performance, SLOF Adaptive functioning was significantly associated with smoking history (F(2,754)=5.3, p=0.005), with post-hoc tests indicating poorer functioning among current smokers, persisting after adjustment for demographic variables (F (2, 690)=3.6, p=0.025). There were no significant diagnosis-by-smoking history interaction effects on functional capacity. Current smokers performed more poorly than did ex-smokers or never smokers on post-hoc tests. Additional adjustment for symptoms and substance use led to the effect of smoking history no longer being significant (F (2, 674)=2.2, p=0.103). Although the UPSA-B was significantly associated with smoking history in an unadjusted model (F (2, 759)=3.5, p=0.030, this association was no longer significant when adjusted for demographic or symptom variables. Pack years were negatively associated with SLOF scores after adjusting for demographic and clinical variables (r2 change =0.025,, F 1, 277=13.1, p<0.001). However, this effect was not significant for UPSA scores. Neither SLOF nor UPSA scores were associated with time since stopped smoking among ex-smokers.
DISCUSSION
This study investigated the association between smoking history and intensity with cognitive and adaptive functioning in a comparatively large and homogenous sample of patients with bipolar disorder or schizophrenia. Several findings contribute to the understanding of smoking and its relationship with cognitive and adaptive functioning. Consistent with the findings of prior epidemiological studies (2), patients with schizophrenia in the current study had substantially greater lifetime exposure to smoking, in terms of the rate of current smoking, number of cigarettes smoked per day, and pack years accumulated across the lifespan than patients with bipolar disorder. Contrary our hypotheses based on some past studies in SMI, current smokers performed worse than past or never smokers; the effect size contrasting current and never smokers was clinically significant (Cohen’s d=0.41). These effects persisted after adjusting for demographic variables that were associated with smoking histories, although were not significant once adjusted for symptom or substance use comorbidity. Current smokers with bipolar disorder and schizophrenia were similar in the severity of their cognitive impairments, which was in notable contrast to never and prior smokers. Thus, our results are consistent with prior research indicating a negative association between smoking and cognition in serious mental illnesses (16) (17).
Even in this relatively highly educated sample that was experiencing a low level of symptoms on average, history of smoking was associated with variation in current symptoms and substance abuse. In particular, patients who reported currently smoking were more likely to exhibit positive symptoms in both diagnoses, and also report higher frequency of use of alcohol and illicit substance usage. Indeed, the impact of current smoking on cognitive and functional outcomes was mitigated by symptom and substance use comorbidity. These illness associations highlight the need for studies to examine the influence of demographic and symptom covariates when investigating the association of smoking and cognitive function. The mechanism explaining the negative association between current smoking and cognitive/adaptive functioning is unclear. One possibility is that an underlying predisposition to nicotine addiction may at least partially overlap with neurobiological mechanisms of cognitive and adaptive impairments in SMI (e.g., alternations in dopaminergic systems). Another non-mutually exclusive explanation is that current smoking is a marker for “insulation” from the societal trend of declining rates of smoking – those patients who continue to smoke may be less susceptible to societal influences that promote abstinence from smoking, perhaps as a result of functional and cognitive impairments.
Interestingly, although current smokers were more cognitively and functionally impaired than past or never smokers, the distinctions between past and never smokers were somewhat less clear. On one hand, investigation of possible linear and non-linear dose effects of smoking intensity (i.e., pack years, cigarettes per day) indicated a negative association with greater cognitive impairment associated with greater pack years and smoking intensity as indicated by cigarettes per day. On the other hand, no pairwise significant differences between ex-smokers and never smokers emerged. One would expect ex-smokers (who reported an average of 18 to 25 pack years of smoking) to exhibit worse cognitive performance than never smokers. It may be that deleterious effects of smoking on cognition may reverse after stopping smoking, although lifetime exposure models combining ex- and current smokers seemed to indicate a linear and negative association between worse cognition and smoking intensity, even after adjusting for age.
Although the sample was comparatively large compared to prior samples, these findings must be taken in light of the limitations of this study. Chiefly, the rate of smoking was substantially lower in this sample when compared to prior reported data(1). This suggests that aspects of the sampling frame are associated with the rate of smoking, in particular that this sample was comprised of a single ethnic group with a low rate of substance abuse comorbidities and mild severity of symptoms. We speculate that greater symptom severity may override the impact of smoking in more severely ill samples, although it is possible that more severely ill samples would have a greater lifetime exposure to smoking and therefore may experience greater impact on cognition and function. Relatedly, we lacked a normal comparison sample, and so we are not able to determine if the sample population’s specific association between smoking and cognitive performance. Thus, the results, in particular the frequency of smoking, may fail to generalize to the broader population of patients with serious mental illnesses. Another limitation is that these data were cross-sectional, disallowing the any interpretation of causal influences among smoking, cognition, and adaptive function. We used diagnoses from the baseline (parent study) and so cannot rule change in diagnoses at the time of cognitive and functional testing. Smoking data was obtained via a validated questionnaire without objective measures of biomarkers of nicotine, which may have provided for a clearer picture of the association between nicotine and cognitive and adaptive functioning in this population. Time of the most recent nicotine use was not measured at the time of cognitive testing, and so it is unclear whether withdrawal symptoms impacted cognitive performance. Our cognitive test battery was ill equipped to examine sub-domains of cognition, which should be a focus for future study. These large samples were associated with statistical significance for relatively small-medium effect sizes associated with smoking. Finally, we lacked a standardized measure of manic symptoms, and so we cannot address the impact of manic symptoms on smoking behavior and history.
Despite these limitations, our findings have several implications with respect to the relevance of smoking behavior to cognitive and adaptive functioning in serious mental illness. Smoking history was associated with cognitive performance even after considering demographic covariates. In particular, current smokers fared worse on standardized cognitive measures as well as functional outcome; performance-based functional capacity was likewise worse among smokers although this did not persist after demographic adjustment. It is unclear if stopping smoking would mitigate these impairments evidenced by current smokers, but some optimism comes from the finding that ex-smokers were not differentiable from never smokers in terms of cognitive or adaptive function. Our results suggest that current cigarette smoking may be associated with worse cognitive and adaptive functioning and should be treated as risk factor for poor cognitive health and functional impairment in SMI, in addition to many other health problems. Finally, it is worth noting that smoking cessation programs drawn from the general population appear equally effective among people with SMI(1, 37).
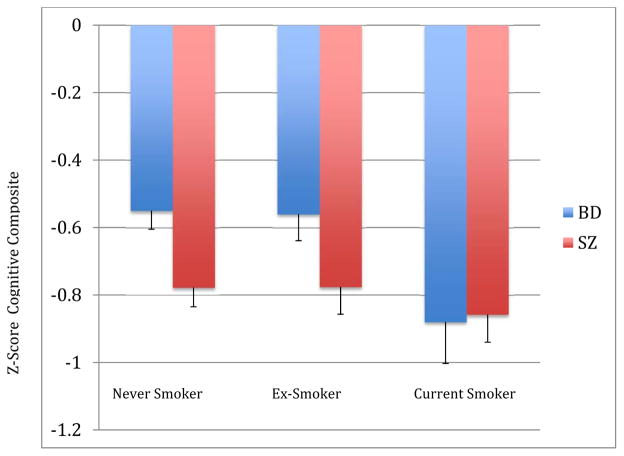
Figure 1. Estimated Marginal Means for the Cognitive Composite by Smoking Status Stratified by Diagnosis.
Adjusted for age, sex, independent living status, education, PANSS Positive Syndrome Scale, PANSS Negative Syndrome Scale, BDI Total Score, Khavari Alcohol Index, and Past month illicit substance use; (F, 2,730)=3.1, p=0.045, post-hoc (Tukey) Current smokers < Never Smokers
Acknowledgments
Supported by NIMH grant R01MH100417 to Dr. Depp, R01MH079784 to Dr. Pulver, grant RO1MH78775 to Dr. Harvey, and R01MH078737 to Dr. Patterson
Footnotes
Declaration of Interest
In the past two years, Dr. Bowie has served on a scientific advisory board and as a consultant for Abbott Pharmaceuticals. Dr. Harvey has served as a consultant to Abbvie, Boeheringer-Ingelheim, En Vivo, Genentech, Otsuka-America, Roche Pharma, Sunovion Pharma, and Takeda Pharma. Authors Depp, Mausbach, Patterson, Wolyneic, Thornquist, Luke, McGrath, and Pulver have no financial relationships with commercial interests.
References
- 1.DE LEON J, DIAZ FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schiz res. 2005;76:135–57. doi: 10.1016/j.schres.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 2.DICKERSON F, STALLINGS CR, ORIGONI AE, et al. Cigarette smoking among persons with schizophrenia or bipolar disorder in routine clinical settings, 1999–2011. Psychiatr Serv. 2013;64:44–50. doi: 10.1176/appi.ps.201200143. [DOI] [PubMed] [Google Scholar]
- 3.CENTERS FOR DISEASE CONTROL. Current Cigarette Smoking Among Adults—United States, 2011. JAMA. 2012:889–994. [PubMed] [Google Scholar]
- 4.AUBIN H-J, ROLLEMA H, SVENSSON TH, WINTERER G. Smoking, quitting, and psychiatric disease: a review. Neurosci Biobehav Rev. 2012;36:271–84. doi: 10.1016/j.neubiorev.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 5.CHANG C-K, HAYES RD, PERERA G, et al. Life expectancy at birth for people with serious mental illness and other major disorders from a secondary mental health care case register in London. PloS one. 2011;6:e19590. doi: 10.1371/journal.pone.0019590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.SABIA S, ELBAZ A, DUGRAVOT A, et al. Impact of smoking on cognitive decline in early old age: The Whitehall II cohort study. Arch Gen Psychiatr. 2012;69:627–35. doi: 10.1001/archgenpsychiatry.2011.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MONS U, SCHOTTKER B, MULLER H, KLIEGEL M, BRENNER H. History of lifetime smoking, smoking cessation and cognitive function in the elderly population. European J Epidem. 2013;28:823–31. doi: 10.1007/s10654-013-9840-9. [DOI] [PubMed] [Google Scholar]
- 8.SWAN GE, LESSOV-SCHLAGGAR CN. The effects of tobacco smoke and nicotine on cognition and the brain. Neuropsychol rev. 2007;17:259–73. doi: 10.1007/s11065-007-9035-9. [DOI] [PubMed] [Google Scholar]
- 9.MORISANO D, WING VC, SACCO KA, ARENOVICH T, GEORGE TP. Effects of tobacco smoking on neuropsychological function in schizophrenia in comparison to other psychiatric disorders and non-psychiatric controls. Am J Addict. 2013;22:46–53. doi: 10.1111/j.1521-0391.2013.00313.x. [DOI] [PubMed] [Google Scholar]
- 10.GOLDBERG JF, ROY CHENGAPPA K. Identifying and treating cognitive impairment in bipolar disorder. Bipolar Disord. 2009;11:123–37. doi: 10.1111/j.1399-5618.2009.00716.x. [DOI] [PubMed] [Google Scholar]
- 11.LAW CW, SOCZYNSKA JK, WOLDEYOHANNES HO, MIRANDA A, BROOKS JO, III, MCINTYRE RS. Relation between cigarette smoking and cognitive function in euthymic individuals with bipolar disorder. Pharm Biochem Behav. 2009;92:12–6. doi: 10.1016/j.pbb.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 12.ENJUTO S. Cognitive performance and cigarette smoking in first-episode psychosis. European arch of psychiatr clin neurosci. 2009;259:65–71. doi: 10.1007/s00406-008-0835-6. [DOI] [PubMed] [Google Scholar]
- 13.MACKOWICK KM, BARR MS, WING VC, RABIN RA, OUELLET-PLAMONDON C, GEORGE TP. Neurocognitive endophenotypes in schizophrenia: Modulation by nicotinic receptor systems. Prog Neuro-Psychopharm Biol Psychiatr. 2013 doi: 10.1016/j.pnpbp.2013.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.SHIM J-C, JUNG D-U, JUNG S-S, et al. Adjunctive varenicline treatment with antipsychotic medications for cognitive impairments in people with schizophrenia: a randomized double-blind placebo-controlled trial. Neuropsychopharm. 2011;37:660–8. doi: 10.1038/npp.2011.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.ZHANG X, CHEN D, XIU M, et al. Cigarette smoking, psychopathology and cognitive function in first-episode drug-naive patients with schizophrenia: a case-control study. Psychol Med. 2012;13:1–10. doi: 10.1017/S0033291712002590. [DOI] [PubMed] [Google Scholar]
- 16.LEVANDER S, EBERHARD J, LINDSTROM E. Nicotine use and its correlates in patients with psychosis. Acta Psychiatr Scand. 2007;116:27–32. doi: 10.1111/j.1600-0447.2007.01085.x. [DOI] [PubMed] [Google Scholar]
- 17.ZHANG XY, XIU MH, HAILE CN, et al. Cigarette smoking and cognitive function in Chinese male schizophrenia: a case-control study. PloS one. 2012;7:e36563. doi: 10.1371/journal.pone.0036563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.HAHN B, HARVEY AN, CONCHEIRO-GUISAN M, HUESTIS MA, HOLCOMB HH, GOLD JM. A test of the cognitive self-medication hypothesis of tobacco smoking in schizophrenia. Biol psychiatr. 2013;74:436–43. doi: 10.1016/j.biopsych.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DIAZ FJ, JAMES D, BOTTS S, MAW L, SUSCE MT, DE LEON J. Tobacco smoking behaviors in bipolar disorder: a comparison of the general population, schizophrenia, and major depression. Bipolar Disord. 2009;11:154–65. doi: 10.1111/j.1399-5618.2009.00664.x. [DOI] [PubMed] [Google Scholar]
- 20.DEPP CA, MAUSBACH BT, HARMELL AL, et al. Meta-analysis of the association between cognitive abilities and everyday functioning in bipolar disorder. Bipolar Disord. 2012 May;14:217–26. doi: 10.1111/j.1399-5618.2012.01011.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.BOWIE CR, DEPP C, MCGRATH JA, et al. Prediction of real-world functional disability in chronic mental disorders: a comparison of schizophrenia and bipolar disorder. Am J Psychiatr. 2010 Sep;167:1116–24. doi: 10.1176/appi.ajp.2010.09101406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.BRAY SM, MULLE JG, DODD AF, PULVER AE, WOODING S, WARREN ST. Signatures of founder effects, admixture, and selection in the Ashkenazi Jewish population. Proc Natl Acad Sci. 2010 Sep 14;107:16222–7. doi: 10.1073/pnas.1004381107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NURNBERGER JI, JR, BLEHAR MC, KAUFMANN CA, et al. Diagnostic Interview for Genetic Studies: Rationale, Unique Features, and Training. Arch Gen Psychiatry. 1994 Nov 1;51:849–59. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- 24.HARVEY PD, HEATON RK, CARPENTER WT, JR, GREEN MF, GOLD JM, SCHOENBAUM M. Diagnosis of schizophrenia: Consistency across information sources and stability of the condition. Schiz res. 2012;140:9–14. doi: 10.1016/j.schres.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.CUMMING AG, MATTHEWS NL, PARK S. Olfactory identification and preference in bipolar disorder and schizophrenia. European arch psychiatry neurosciences. 2011;261:251–9. doi: 10.1007/s00406-010-0145-7. [DOI] [PubMed] [Google Scholar]
- 26.VARTIAINEN E, SEPPALA T, LILLSUNDE P, PUSKA P. Validation of self reported smoking by serum cotinine measurement in a community-based study. J Epidem Comm Health. 2002;56:167–70. doi: 10.1136/jech.56.3.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MORABIA A, BERNSTEIN MS, CURTIN F, BERODE M. Validation of self-reported smoking status by simultaneous measurement of carbon monoxide and salivary thiocyanate. Prev med. 2001;32:82–8. doi: 10.1006/pmed.2000.0779. [DOI] [PubMed] [Google Scholar]
- 28.MAUSBACH BT, HARVEY PD, GOLDMAN SR, JESTE DV, PATTERSON TL. Development of a brief scale of everyday functioning in persons with serious mental illness. Schiz Bull. 2007;33:1364–72. doi: 10.1093/schbul/sbm014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.SCHNEIDER LC, STRUENING EL. SLOF: a behavioral rating scale for assessing the mentally ill. Soc Work Res Abstr. 1983 Fall;19:9–21. doi: 10.1093/swra/19.3.9. [DOI] [PubMed] [Google Scholar]
- 30.BOWIE CR, LEUNG WW, REICHENBERG A, et al. Predicting schizophrenia patients’ real-world behavior with specific neuropsychological and functional capacity measures. Biol Psychiatr. 2008 Mar 1;63:505–11. doi: 10.1016/j.biopsych.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.BOWIE CR, REICHENBERG A, PATTERSON TL, HEATON RK, HARVEY PD. Determinants of real-world functioning performance in Schizophrenia: Correlations with cognition, functional capacity, and symptoms. Am J psychiatr. 2006;163:418–25. doi: 10.1176/appi.ajp.163.3.418. [DOI] [PubMed] [Google Scholar]
- 32.BOWIE CR, TWAMLEY EW, ANDERSON H, HALPERN B, PATTERSON TL, HARVEY PD. Self-assessment of functional status in schizophrenia. J Psychiatric Res. 2007 Dec;41:1012–8. doi: 10.1016/j.jpsychires.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DEPP C, STRASSNIG M, BOWIE C, et al. Association of BMI and cardiometabolic risk factors with cognition in serious mental illness. Bipolar Disord. In Press. [Google Scholar]
- 34.CHIOLERO A, FAEH D, PACCAUD F, CORNUZ J. Consequences of smoking for body weight, body fat distribution, and insulin resistance. Am J Clin Nutr. 2008 Apr 1;87:801–9. doi: 10.1093/ajcn/87.4.801. [DOI] [PubMed] [Google Scholar]
- 35.KHAVARI KA, FARBER PD. A profile instrument for the quantification and assessment of alcohol consumption. The Khavari Alcohol Test. J Stud Alcohol. 1978 Sep;39:1525–39. doi: 10.15288/jsa.1978.39.1525. [DOI] [PubMed] [Google Scholar]
- 36.DOZOIS DJA, DOBSON KS, AHNBERG JL. A psychometric evaluation of the Beck Depression Inventory – II. Psychol Assess. 1998;10:83–9. [Google Scholar]
- 37.KAY S, OPLER L, LINDENMAYER J. Reliability and validity of the Positive and Negative Syndrome Scale for schizophrenia. Schiz Bull. 1988;13:261–76. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 38.BANHAM L, GILBODY S. Smoking cessation in severe mental illness: what works? Addiction. 2010;105:1176–89. doi: 10.1111/j.1360-0443.2010.02946.x. [DOI] [PubMed] [Google Scholar]
- 39.VAN HASSELT FM, KRABBE PFM, VAN ITTERSUM DG, POSTMA MJ, LOONEN AJM. Evaluating interventions to improve somatic health in severe mental illness: a systematic review. Acta Psychiatr Scand. 2013;128:251–60. doi: 10.1111/acps.12096. [DOI] [PubMed] [Google Scholar]