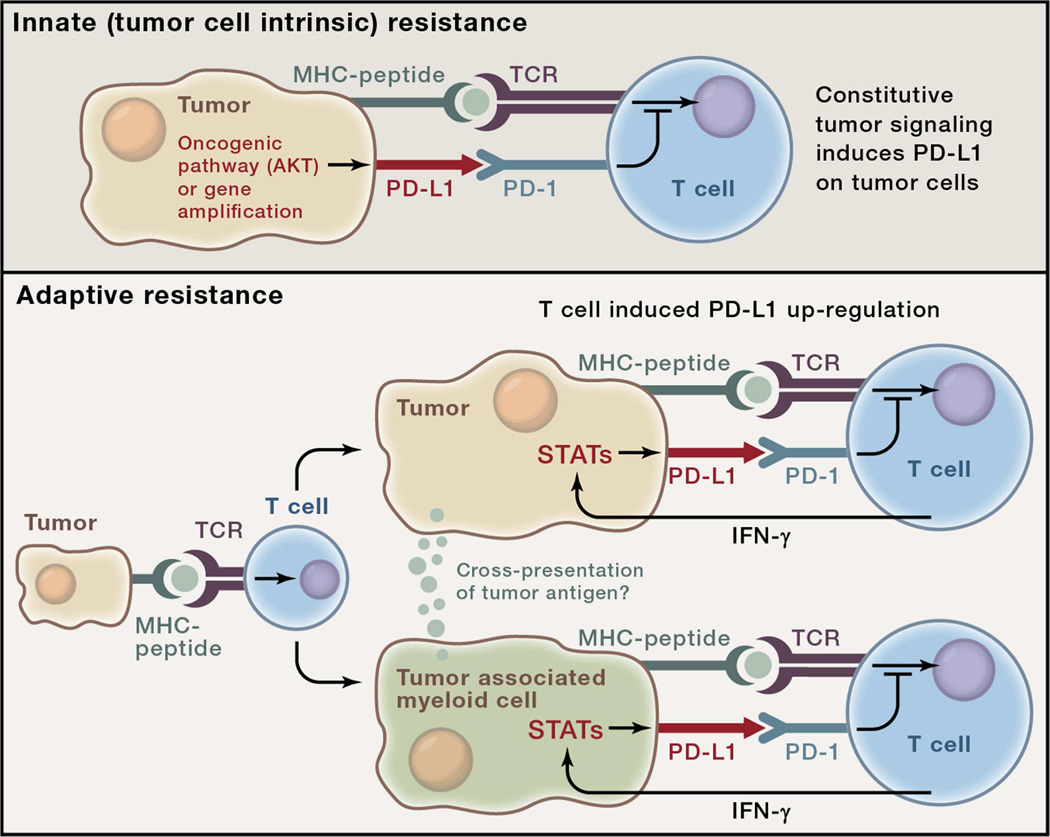
Figure 2. Two general mechanisms for expression of checkpoint ligands in the tumor microenvironment (TME).
The examples in this figure use the PD-1 ligand, PD-L1 for illustrative purposes although the concept likely applies to multiple checkpoint ligands. Top: Innate immune resistance. In some tumors, constitutive oncogenic signaling, such as through activation of the AKT pathway or gene amplification, can up-regulate PD-L1 expression on tumor cells independently of inflammatory signals in the TME. Bottom: Adaptive immune resistance refers to PD-L1 induction in tumors as an adaptation to sensing of immune attack. In adaptive resistance, PD-L1 is not constitutively expressed but rather, is induced by inflammatory signals such as IFN-g produced by T cells attempting to execute an active anti-tumor response. Expression of PD-L1 in a non-uniform distribution associated with lymphocyte infiltrates suggests adaptive induction in response to immune reactivity within the TME. Adaptive resistance can be generated by cytokine-induced PD-L1 expression on either tumor cells themselves or on leukocytes (macrophages, myeloid suppressor cells, dendritic cells or even lymphocytes) in the TME. Inhibition of tumor specific T cells by PD-L1- (or PD-L2)-expressing leukocytes may involve cross-presentation of tumor antigens such that PD-1-dependent inhibition is in cis. Adaptive resistance may be a common mechanism for the intratumoral expression of multiple immune checkpoint molecules.