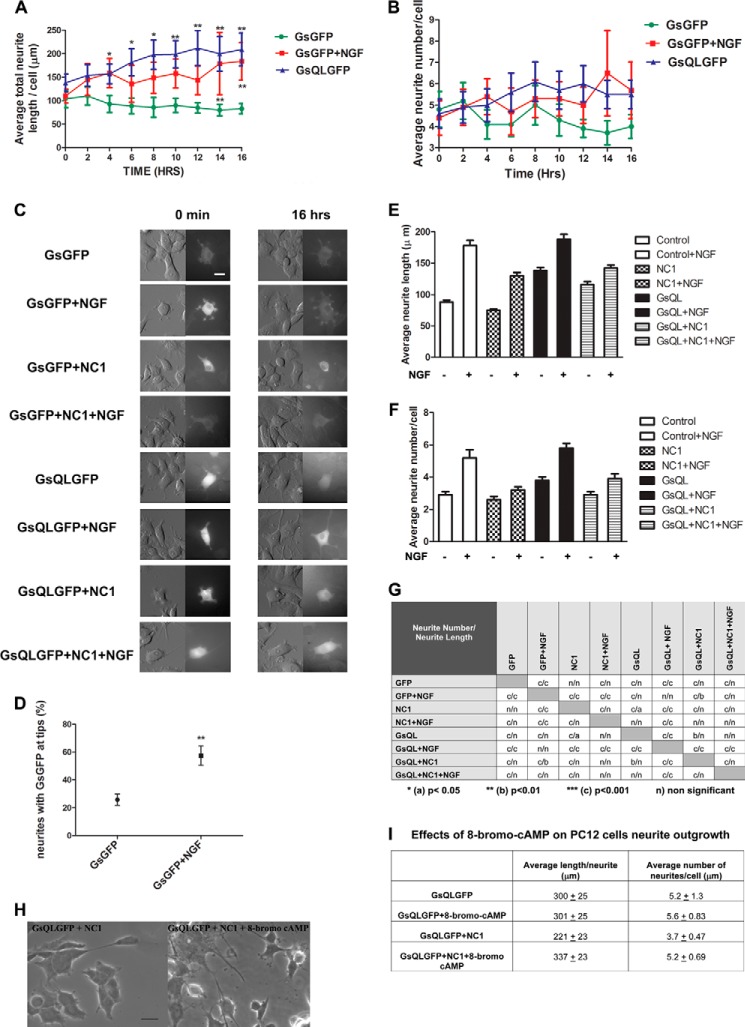
FIGURE 4.
NGF and constitutively activated GsQL increase neurite length and number in PC12 cells, and this is inhibited by interference with Gαs association with tubulin. PC12 cells were electroporated with GsGFP (control), GsQLGFP (constitutively active GαsQ227L), NC1 (a dominant-negative G protein chimera that inhibits GFP-Gαs association with tubulin) with GsGFP and GsQLGFP with NC1. Cells were induced with NGF after 24 h of protein expression where indicated. Live cell imaging was performed using the VivaView system (see “Experimental Procedures”). C, cells were imaged under DIC and fluorescence (GFP) for 16 h. We identified transfected cells by the intensity of GFP staining. GsQL protein is extensively accumulated in the cell “soma” and in the peripheral regions of the growth cones enriched in actin-based cytoskeleton (21). Images are represented at 0 min and 16 h. Scale bar = 50 μm. A and B, statistical analysis of neuronal growth per cell. Control cells were compared with cells expressing GsQLGFP and GsGFP stimulated with NGF. The average total length (A) and number of neurites (B) were measured. The effect of NGF and GsQL on the number of neurites was insignificant. The data for neurite length were analyzed by column statistics followed by unpaired Student's t test. **, two-tailed p < 0.05. Data are expressed as mean ± S.E. Significance was calculated by Student's t test. **, p < 0.01 (control versus GsQL, time points 10, 14, and 16). One-way ANOVA was used to determine significance among conditions (p = 0.0001). D, statistical analysis of the percentage of neurites expressing GFP at the tips. NGF induced the translocation of Gαs to the neurite tip compared with control cells. Data are mean ± S.E. Significance was calculated by Student's t test. **, two-tailed p <0.05 (control versus GsGFP + NGF). E and F, a dominant-negative G protein chimera that inhibits GFP-Gαs association with tubulin attenuates the number and length in PC12 cells. PC12 cells were induced to differentiate in low-serum medium containing NGF (50 ng/ml) for 4 days. 72 h after plating, cells were transiently transfected with plasmids encoding the control (GFP), a Giα1-Gt chimera that binds to tubulin and blocks the activation of tubulin GTPase by Gαs (NC1), and constitutively active GsQL. We identified NC1-expressing cells by staining with a His tag antibody. E, quantitative analysis of the mean average length per cell. F, average neurite number per cell. Transfections were repeated at least three times with 120–180 cells scored per conditions in each experiment. Error bars represent S.E. G, statistical analysis of mean average neurite number and length per cell. Two-way ANOVA followed with Bonferroni post test was used to determine statistical significance among conditions. H and I, effects of 8-bromo-cyclic AMP on PC12 cells expressing GsQLGFP and NC1. PC12 cells were electroporated with GsQLGFP alone or GsQLGFP with NC1 simultaneously (GsQLGFP + NC1) and treated or untreated with 8-bromo-cyclic AMP (100 μm) for 48 h. H, images were taken at ×40 magnification using a Zeiss AX10 microscope system. Scale bar = 20 μm. I, the average total length and number of neurites were measured. For the analysis, 11 cells were selected from the untreated group (−8-bromo-cyclic AMP) and 14 cells from the group treated with 8-bromo-cyclic AMP (+8-bromo-cyclic AMP). The effect of 8-bromo-cyclic AMP on the number of neurites was insignificant (p = 0.109). The data for neurite length were analyzed by column statistics followed by Student's t test (two-tailed p < 0.05). Data are expressed as mean ± S.E. Significance was calculated by Student's t test (p = 0.0014 with or without 8-bromo-cyclic AMP).