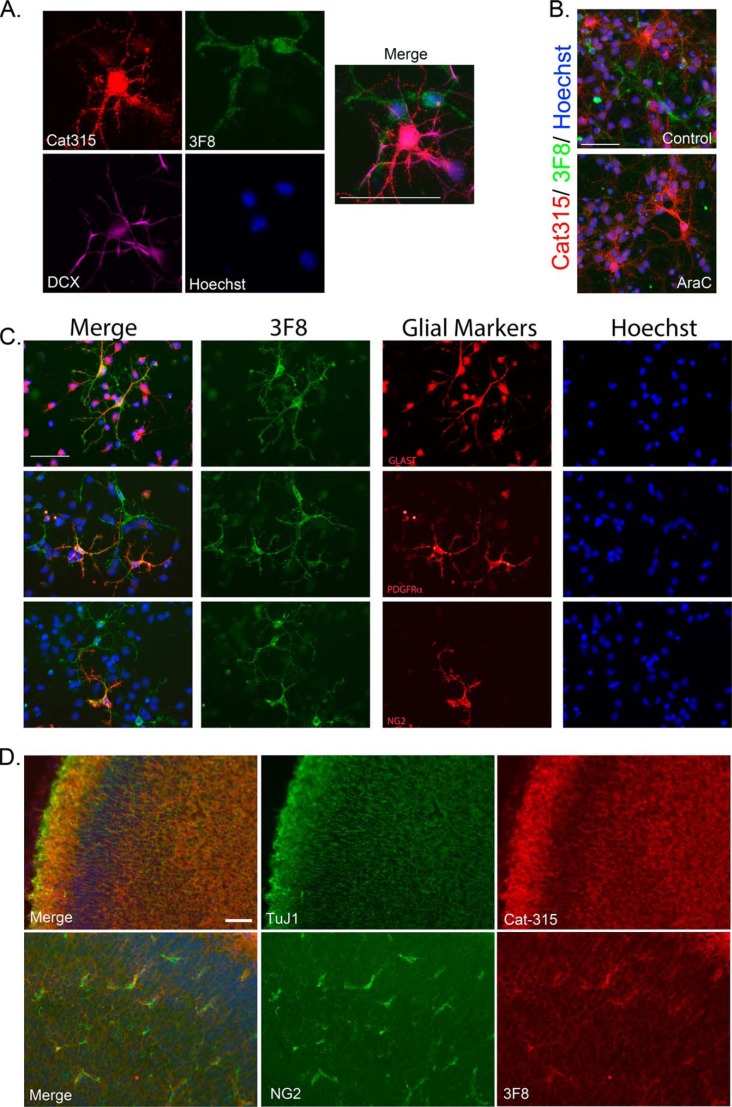
FIGURE 2.
3F8 reactive forms of RPTPζ/phosphacan decorate glial subclasses. Primary mixed neural cell cultures were generated from the cortex of embryonic day 16 animals and stained for antibodies against O-mannosyl-linked carbohydrate epitopes on RPTPζ/phosphacan. A, cultures were fixed at 4 DIV and triple-stained for Cat-315 (red), 3F8 (green), and DCX (violet). Whereas Cat-315 immunoreactivity was confirmed to be localized to DCX-positive neurons, 3F8-immunoreactive cells were not DCX-positive. B, cultures were treated with 5 μm AraC from 1 to 3 DIV to remove actively dividing cells and fixed at 4 DIV. Cultures were then double-stained for Cat-315 (red) and 3F8 (green). In untreated control cultures, populations of cells reactive for Cat-315 and 3F8 were present. Following AraC treatment, cultures retained Cat-315 immunoreactivity but lost 3F8 immunoreactivity. C, cultures were fixed at 4 DIV and double-stained for 3F8 and markers of glial cell subclasses, including glial glutamate transporter GLT-1 (GLAST), platelet-derived growth factor receptor α (PDGFRα), and NG2 chondroitin sulfate proteoglycan (NG2). Cells decorated with 3F8 immunoreactivity were reactive for glial cell class markers GLAST, PDGFRα, and NG2. D, tissue sections from P0 brains stained with the neuronal marker TuJ1 and Cat-315 demonstrate that Cat-315-reactive phosphacan is associated with neuronal cells and processes. In contrast, sections stained with NG2 and 3F8 demonstrate that 3F8-reactive phosphacan is enriched around a subset of glial cells. Together, these data reveal the cell-specific expression of different phosphacan glycoforms. In all cases, cells are counterstained with Hoechst nuclear stain (blue). Scale bar, 50 μm.