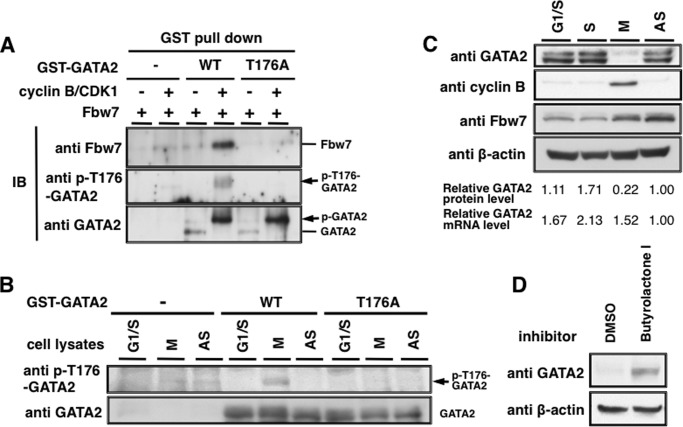
FIGURE 6.
Phosphorylation of Thr176 of GATA2 participates in binding with Fbw7 and GATA2 is decreased in M phase. A, Thr176-phosphorylated GATA2 binds Fbw7 in vitro. Purified GST-fused WT- or T176A-GATA2 or GST protein using glutathione beads was incubated with or without recombinant cyclin B1-CDK1 in reaction buffer containing 1 mm ATP for 30 min. The reaction mixtures were incubated with lysates from HEK293 cells expressing FLAG-Fbw7 and then precipitated using glutathione-Sepharose beads and subjected to IB with the indicated antibodies. B, Thr176 of WT-GATA2 protein was phosphorylated by cell lysates prepared from synchronized HeLa cells at the M phase in vitro. Purified GST-fused WT- or T176A- GATA2 or GST proteins with glutathione beads were incubated with lysates prepared from HeLa cells arrested in G1/S or M phase or asynchronized (AS) HeLa cells in reaction buffer containing 1 mm ATP for 30 min. The mixtures were treated with precision protease for 30 min to cleave out GATA2 from the GST tag and then subjected to immunoblotting with indicated antibodies. C, endogenous GATA2 is decreased in the M phase. Lysates prepared from HeLa cells arrested at G1/S, S, or M phase or asynchronized HeLa cells were subjected to immunoblotting with indicated antibodies. mRNA levels in synchronized cells were measured by RT-qPCR. Total RNA was isolated from cells and subjected to reverse transcription with random hexanucleotide primers. The resulting complementary DNA was subjected to RT-qPCR. The amount of transcripts was normalized against that of 18 S rRNA as an internal standard. Relative protein and mRNA levels of GATA2 are indicated below. D, effect of CDK inhibitor treatment on GATA2 levels in M phase cells. HeLa cells synchronized at M phase were treated with 10 μm butyrolactone I for 6 h, and then GATA2 levels were analyzed by immunoblotting.