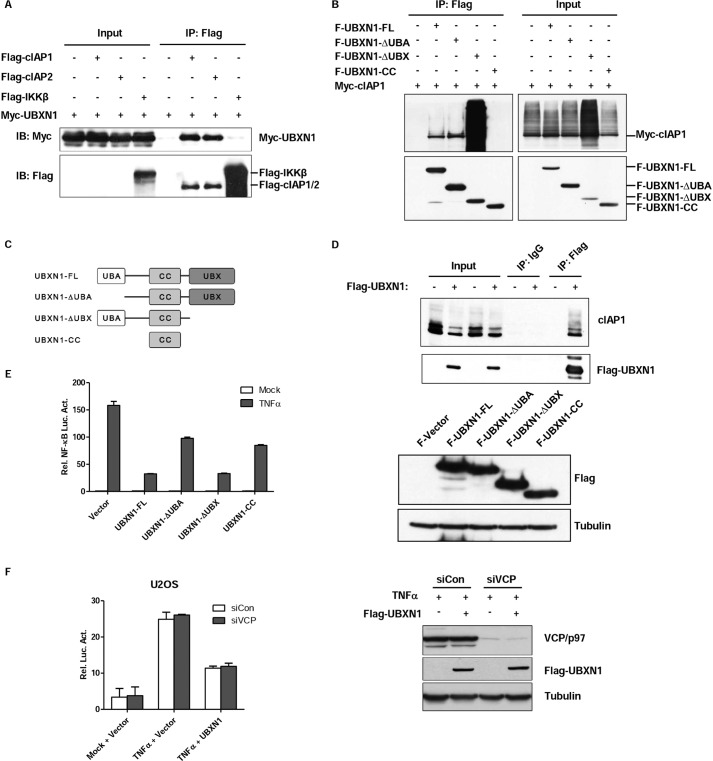
FIGURE 5.
UBA domain of UBXN1 is crucial for UBXN1-mediated NF-κB inhibition. A–D, UBXN1 interacts with cIAPs. A, immunoassay of lysates of HEK293T cells cotransfected with vectors expressing FLAG-tagged (Flag-) cIAP1, cIAP2, or IKKβ and Myc-tagged (Myc-) UBXN1, immunoprecipitated (IP) with anti-FLAG, and analyzed by immunoblotting (IB) with anti-Myc. B, UBXN1 interacts with cIAP1 through either the UBA domain or UBX domain. Coimmunoprecipitation of cIAP1 and UBXN1 from HEK293T cells expressing Myc-cIAP1 and FLAG-tagged UBXN1 mutants using anti-FLAG-agarose beads was followed by immunoblot using a FLAG or Myc antibody. C, schematic representation of UBXN1 deletion mutants. D, immunoblot analysis of the interaction between endogenous cIAP1 proteins and exogenous UBXN1 in lysates of HEK293T cells transfected with either an empty vector or FLAG-UBXN1 plasmid after immunoprecipitation with mouse IgG or anti-FLAG (right) or with the same amount of cell lysate used for immunoprecipitation (Input; left). E, UBA domain of UBXN1 is essential for inhibiting TNFα-triggered NF-κB activation. Dual-luciferase assays of NF-κB activity in TNFα-treated HEK293T cells overexpressing FLAG-tagged UBXN1 truncates as described in C. The expression levels of FLAG-UBXN1 truncated mutants were examined by immunoblots. F, knockdown of VCP/p97 does not have effect on UBXN1-mediated NF-κB inhibition. The experiment was similarly performed as in Fig. 2A.