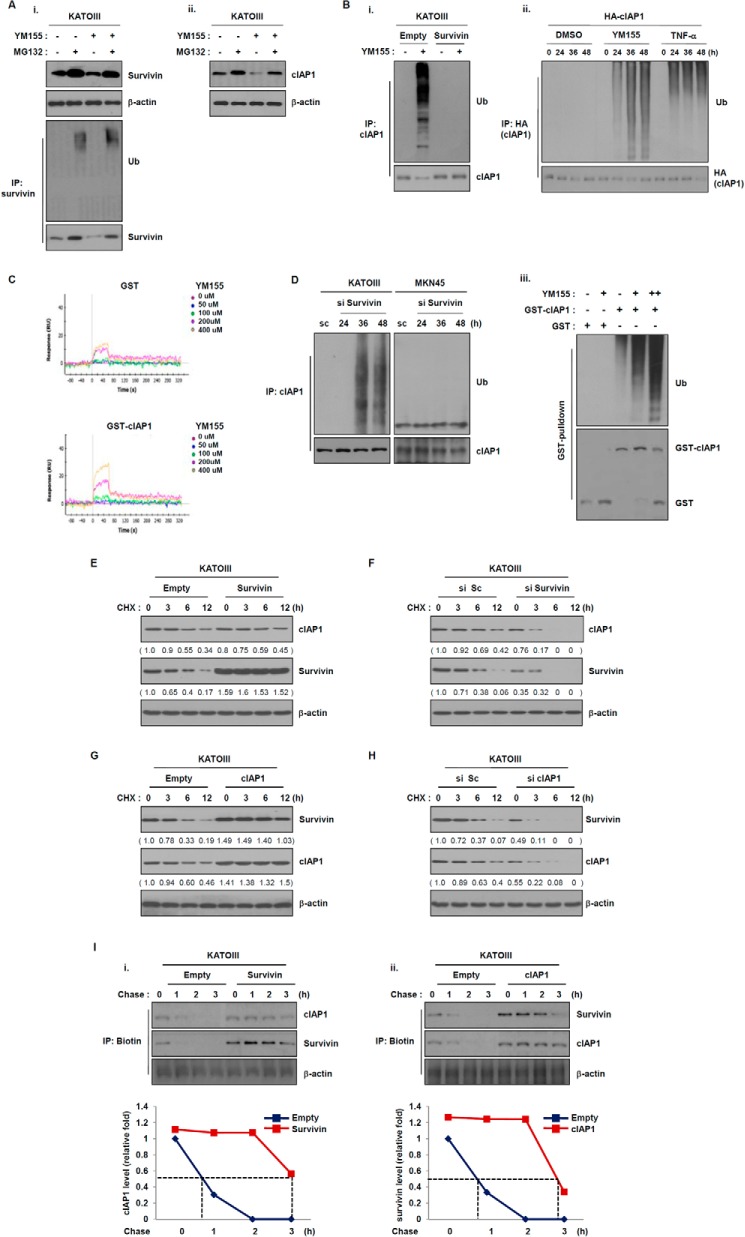
FIGURE 4.
Survivin regulates the stability of cIAP1 in a proteasome-dependent manner. A, KATOIII cells were treated YM155 (20 nm) for 48 h and then incubated with the proteasome inhibitor MG132 (25 μm) for 6 h. (i) Survivin and (ii) cIAP1 expressions were determined by Western blot analysis. B, (i) KATOIII cells were transfected with a GFP-tagged survivin plasmid for 48 h and then treated with YM155 (20 nm) for another 48 h. Cell lysates were immunoprecipitated with anti-cIAP1 and Western blot analysis as performed with an anti-ubiquitin (Ub) antibody. (ii) 293T cells were transfected with HA-tagged cIAP1 plasmid for 24 h and then treated with YM155 (20 nm) or TNF-α (100 ng/ml). The cell lysates were immunoprecipiated with anti-HA antibody. The ubiquitination of cIAP1 by YM155 was determined via Western blot analysis using anti-ubiquitin antibody. (iii) Recombinant GST-cIAP1 proteins were incubated with E1, E2, ATP, YM155 (10 and 20 nm), and then the ubiquitination of cIAP1 by YM155 was determined with GST pulldown analysis. C, direct interaction of YM155 to cIAP1 using surface plasmon resonance. Representative sensograms were obtained from injections of YM155 at the indicated doses. GST serves as a control to GST-cIAP1. D, cells were transfected with survivin siRNA for the indicated times. Cell lysates were prepared for immunoprecipitation (IP), and cIAP1 ubiquitination was detected using an anti-ubiquitin antibody. E and F, KATOIII cells were transfected with a control vector or a survivin expression vector and scramble or survivin siRNA for 48 h and then treated with the protein synthesis inhibitor cycloheximide (CHX) (50 μg/ml) for the indicated times. Survivin and cIAP1 expression levels were determined by Western blot analysis with anti-survivin and anti-cIAP1 antibodies. G and H, KATOIII cells were transfected with a empty or a cIAP1 expressing plasmid and scramble cIAP1 siRNA for 48 h and then treated with cycloheximide for the indicated times. Cell lysates were prepared for Western blot analysis using anti-survivin and anti-cIAP1 antibodies. I, non-radioactive pulse-chase assay of survivin or cIAP1 protein. KATOIII cells were transfected with empty vector and survivin or cIAP1 expressing plasmid for 48 h. The cells were depleted with methionine-free medium for 1 h and then chased for the indicated times. Relative survivin or cIAP1 protein levels were quantified.