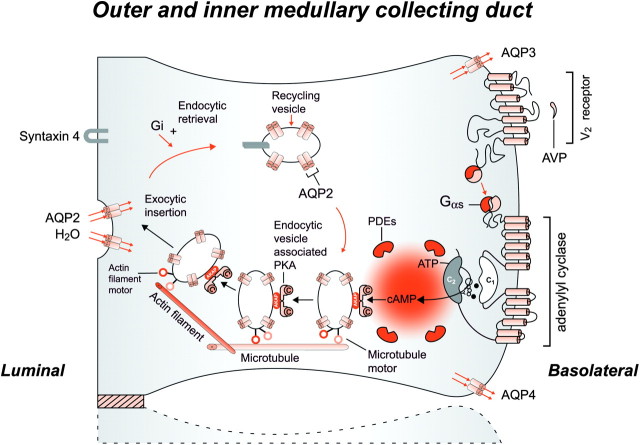
Fig. 5.
Schematic representation of the effect of vasopressin (AVP) to increase water permeability in the principal cells of the collecting duct. AVP is bound to the V2 receptor (a G-protein-linked receptor) on the basolateral membrane. The basic process of G-protein-coupled receptor signaling consists of three steps: a hepta-helical receptor that detects a ligand (in this case, AVP) in the extracellular milieu, a G-protein (Gα s) that dissociates into a subunits bound to guanosine triphosphate and bg subunits after interaction with the ligand-bound receptor and an effector (in this case, adenylyl cyclase) that interacts with dissociated G-protein subunits to generate small molecule second messengers. AVP activates adenylyl cyclase, increasing the intracellular concentration of cAMP. The topology of adenylyl cyclase is characterized by two tandem repeats of six hydrophobic transmembrane domains separated by a large cytoplasmic loop and terminates in a large intracellular tail. The dimeric structure (C1 and C2) of the catalytic domains is represented. Conversion of adenosine triphosphate (ATP) to cAMP takes place at the dimer interface. Two aspartate residues (in C1) coordinate two metal co-factors (Mg2+ or Mn2+ represented here as two small black circles), which enable the catalytic function of the enzyme. Adenosine is shown as an open circle and the three phosphate groups (ATP) are shown as smaller open circles. PKA is the target of the generated cAMP. The binding of cAMP to the regulatory subunits of PKA induces a conformational change, causing these subunits to dissociate from the catalytic subunits. These activated subunits (C) as shown here are anchored to an aquaporin-2 (AQP2)-containing endocytic vesicle via an A-kinase-anchoring protein. The local concentration and distribution of the cAMP gradient are limited by phosphodiesterases (PDEs). Cytoplasmic vesicles carrying the water channels (represented as homotetrameric complexes) are fused to the luminal membrane in response to AVP, thereby increasing the water permeability of this membrane. The dissociation of the A-kinase-anchoring protein from the endocytic vesicle is not represented. Microtubules and actin filaments are necessary for vesicle movement toward the membrane. When AVP is not available, AQP2 water channels are retrieved by an endocytic process, and water permeability returns to its original low rate. Aquaporin-3 (AQP3) and aquaporin-4 (AQP4) water channels are expressed constitutively at the basolateral membrane.