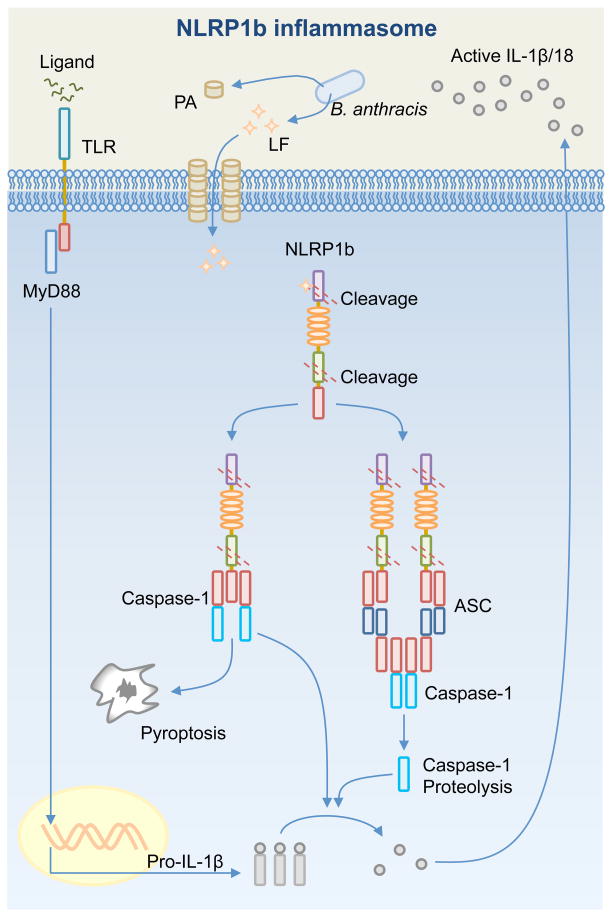
Fig. 2. Activation of the NLRP1b inflammasome.
Bacillus anthracis releases the anthrax lethal toxin, a bipartite toxin composed of a protective antigen (PA) and a lethal factor (LF). PA generates a pore on the host cell membrane, which is used by LF to enter the cell. Inflammasome responds to the presence of LF in the cytosol following LF-induced cleavage of NLRP1b at the N-terminal domain. Autoproteolytic cleavage at the FIIND domain of NLRP1b has also been observed. Cleavage of NLRP1b is sufficient to activate the inflammasome. In response to a high dose of LF, the CARD of NLRP1b binds the CARD of pro-caspase-1. This complex is sufficient to drive pro-IL-1β and pro-IL-18 processing and pyroptosis independently of ASC or caspases-1 self-proteolysis. In response to a low dose of LF, the CARD of NLRP1b recruits ASC to form a macromolecular cytoplasmic speck, where caspase-1 undergoes proteolysis and contributes to pro-IL-1β and pro-IL-18 processing.