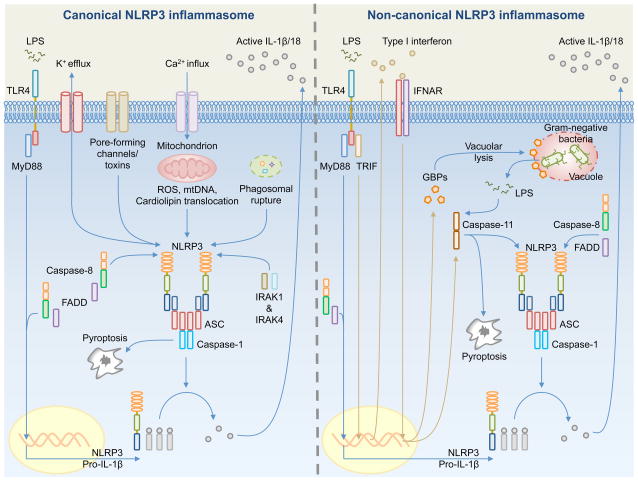
Fig. 3. Mechanisms of activation for the canonical and non-canonical NLRP3 inflammasomes.
(Left). Canonical NLRP3 inflammasome activation requires priming by a Toll-like receptor (TLR) ligand (e.g. LPS) – mediated by MyD88, caspase-8, FADD, and NF-κB – to induce the expression of pro-IL-1β and NLRP3. Pro-IL-18 is expressed constitutively in the cell. A number of mechanisms have been proposed to activate the canonical NLRP3 inflammasome, including K+ efflux, pore-forming channels or toxins, Ca2+ influx, mitochondrial reactive oxygen species (ROS), mitochondrial DNA (mtDNA), translocation of cardiolipin from the inner mitochondrial membrane to the outer mitochondrial membrane, and phagosomal destabilization. NLRP3, ASC and caspase-1 assemble the inflammasome, which leads to proteolytic cleavage of pro-IL-1β and pro-IL-18 for release and the induction of pyroptosis. A priming-independent pathway for canonical NLRP3 inflammasome activation has been described. This pathway requires IRAK1 and IRK4. (Right). Non-canonical NLRP3 inflammasome activation is activated by Gram-negative bacteria. Extracellular LPS induces the expression of pro-IL-1β and NLRP3 via the TLR4-MyD88-dependent pathway and type I interferon via the TLR4-TRIF-dependent pathway. Type I interferon provides a feedback loop and activates type I interferon receptor (IFNAR) to induce caspase-11 expression. Cytosolic Gram-negative bacteria deliver LPS into the cytosol when they escape the vacuole. Vacuolar Gram-negative bacteria release their LPS into the cytosol through a mechanism that requires vacuolar rupture mediated by interferon-inducible guanylate-binding proteins (GBPs). Caspase-11 is proposed to activate following its binding to cytosolic LPS. Caspase-11 then drives pyroptosis and activation of the non-canonical NLRP3 inflammasome.