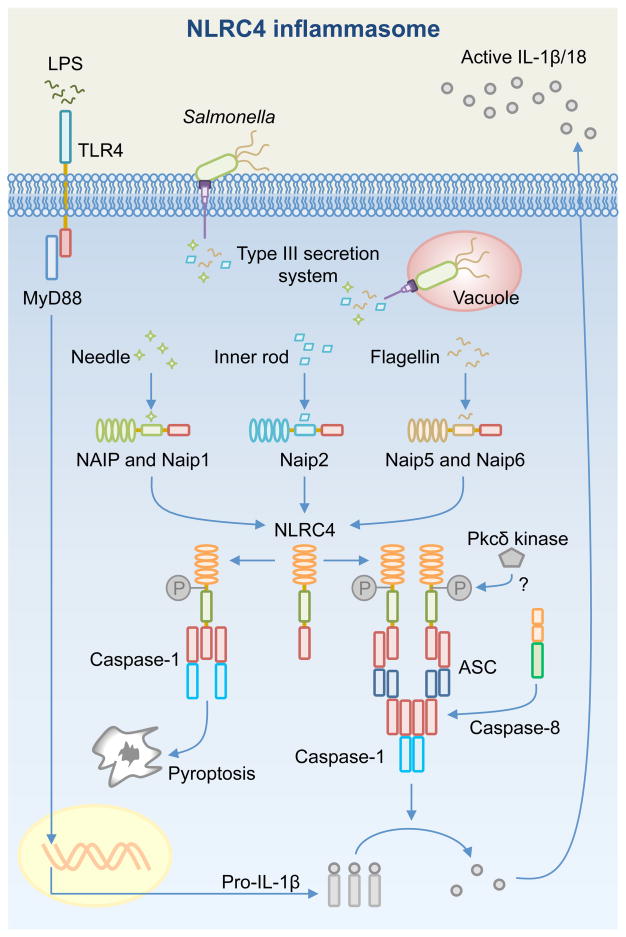
Fig. 4. Activation of the NAIP-NLRC4 inflammasome.
Toll-like receptors (e.g. TLR4) mediate the production of pro-IL-1β via MyD88 and NF-κB. Certain pathogenic bacteria use the Type III secretion system (T3SS) to deliver effector proteins to subvert host cell functions (e.g. Salmonella enterica serovar Typhimurium uses the Salmonella Pathogenicity Island-1 or SPI-1– Type III secretion system). In doing so, bacterial proteins such as the T3SS needle protein, inner rod protein, and flagellin are injected into the cytoplasm. These proteins are detected by NAIPs to activate the NLRC4 inflammasome, which results in pro-IL-1β and pro-IL-18 processing via an ASC-dependent mechanism. Caspase-1 and caspase-8 are recruited to the ASC inflammasome independently of each other during early infection. Caspase-8 is speculated to enhance delayed processing of pro-IL-1β and pro-IL-18 and induces delayed cell death. NLRC4 induces caspase-1-dependent pyroptosis via an ASC-independent mechanism. Phosphorylation of NLRC4 by the Pkcδ kinase is proposed to contribute to the activation of the NLRC4 inflammasome.