Figure 3.
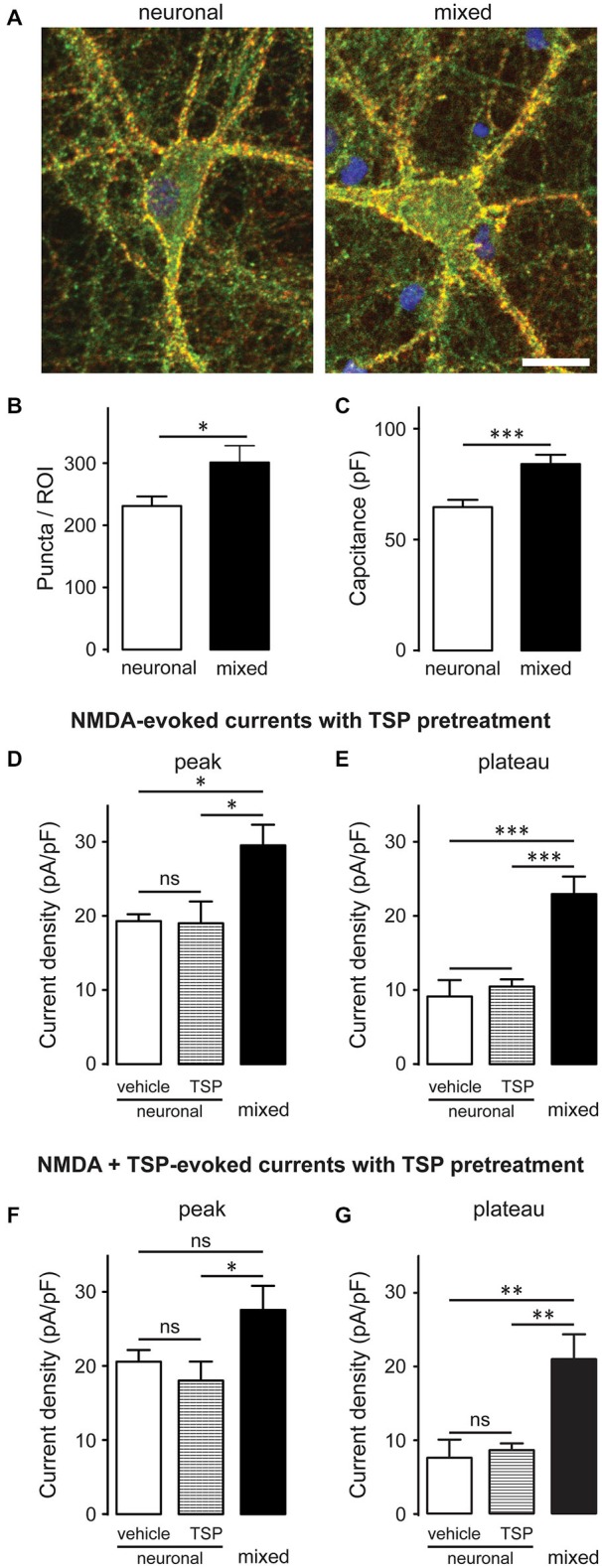
Glia-induced increase in the NMDAR current density is not due to an appreciable change in synaptic density and cannot be mimicked by thrombospondin (TSP) treatment of neuronal cultures. (A) Synaptic puncta were visualized by co-localization of presynaptic marker synaptophysin (red) and the postsynaptic marker PSD-95 (green). Scale bar, 20 μm. (B,C) The number of synaptic puncta per ROI (which included the soma and proximal dendrites of each imaged neuron) was higher in mixed than neuronal cultures (B); however, this increase was proportional to the increase in the neuronal membrane capacitance (a proxy for the neuronal surface area) observed in mixed cultures (C). (B,C: *p < 0.05, ***p < 0.001; t-test) (D,E) 10-day pretreatment of neuronal cultures with 5 μg/ml TSP had no effect on NMDAR current density measured at either peak or plateau time points; for comparison, NMDAR current density in mixed sister cultures was significantly larger than the current density in neuronal cultures after either pretreatment. Control neuronal cultures were pretreated with vehicle (0.07% glycerol); NMDA currents were evoked by application of 200 μM NMDA in the presence of glycine. (F,G) Acute TSP treatment (5 μg/ml) had no effect on NMDAR current density in neuronal cultures. In these experiments, neuronal cultures were first pretreated with 5 μg/ml TSP or vehicle, as described for (D,E); TSP or vehicle were then co-applied with NMDA in the presence of glycine. There was no difference in the mean NMDAR current density between TSP- and vehicle-treated cultures at either time point (peak and plateau); as expected, NMDAR current density was significantly larger in mixed sister cultures. (D–G: *p < 0.05, **p < 0.01, ***p < 0.001, one-way ANOVA with Bonferroni post-test).
