Figure 6.
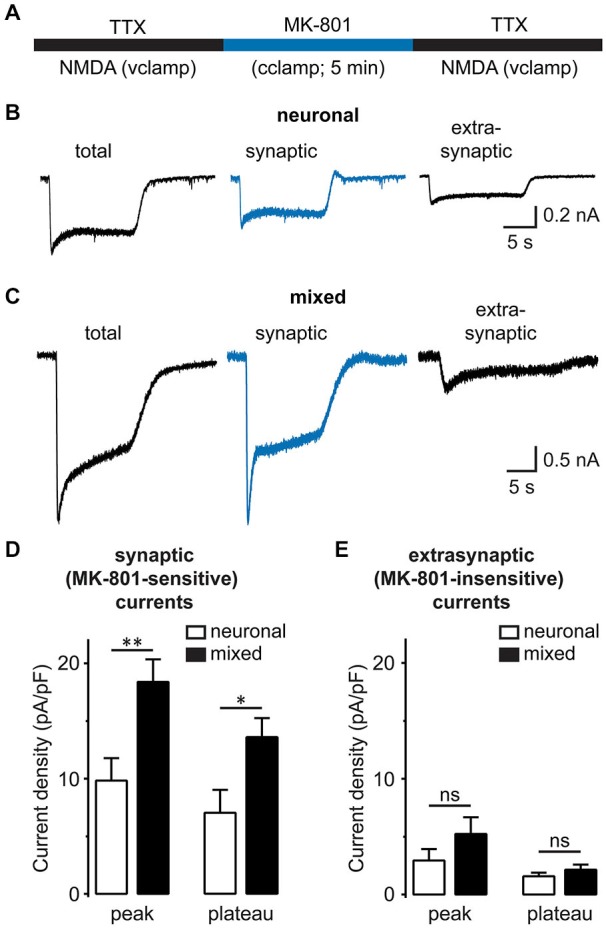
Synaptic, but not extrasynaptic, NMDA-evoked currents are increased in the presence of glia. (A) A schematic diagram of the drug application protocol used to separate synaptic from extrasynaptic NMDAR currents. The total whole-cell NMDAR currents were first recorded in a Mg2+-free external solution containing TTX. To block synaptic NMDA currents, neurons were then switched for 5 min to the current-clamp configuration and a TTX-free solution containing Mg2+ and 10 μM MK-801; under these conditions, the spontaneous neuronal firing activates synaptic (but not extrasynaptic) NMDAR channels, which are then irreversibly blocked by the activity-dependent, irreversible NMDAR channel blocker MK-801. Finally, neurons were switched back to the regular recording solution and voltage clamp configuration to record the residual extrasynaptic NMDA currents. Between patch-clamp recording configuration switches, neurons were perfused for 2 min. (B,C) Representative current traces evoked by 200 μM NMDA in the presence of glycine in neuronal (B) and mixed cultures (C). Total NMDAR currents (left) were recorded before MK-801 treatment, while residual/extrasynaptic NMDAR currents (right) were recorded after MK-801 treatment, as described in (A). Synaptic NMDA currents (center) were obtained by subtracting MK-801-insensitive from total NMDA currents. (D,E) Mean peak and steady-state (plateau) current densities are plotted for synaptic (D) and extrasynaptic NMDA currents (E). The peak current amplitude (averaged over 5 ms) was determined within 1 s of NMDA application; the plateau current amplitude was obtained by averaging during the last 1 s of 10–15 s NMDA application. Both peak and plateau synaptic NMDAR currents were greater in mixed cultures, but extrasynaptic currents did not significantly differ between the two culture conditions (*p < 0.05, **p < 0.01, t-test).
