Figure 5.
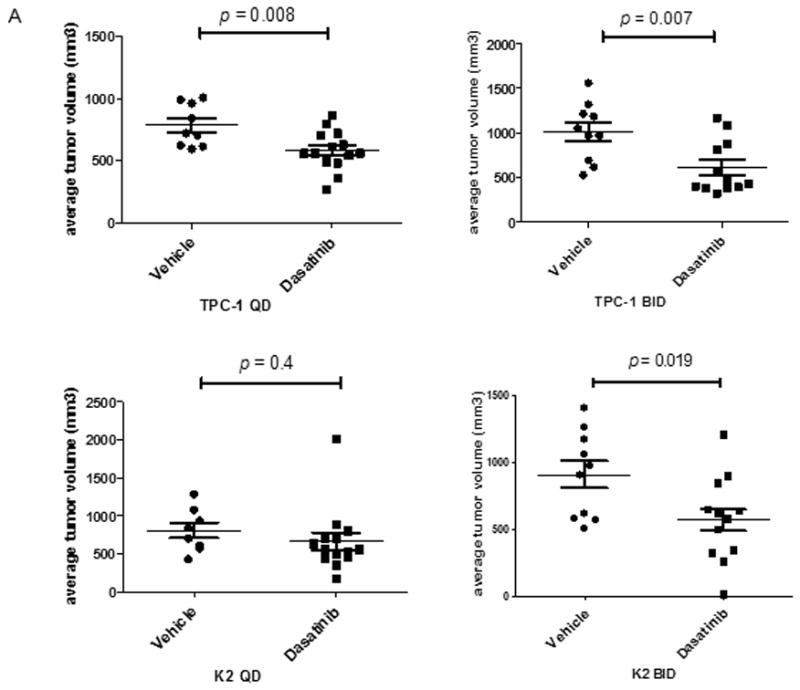
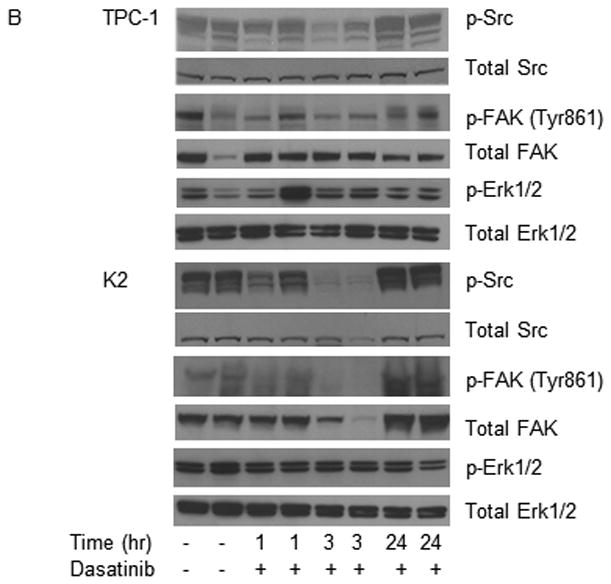
Dasatinib reduces tumor volume in vivo. For the in vivo studies, dasatinib (30 mg/mL) was dissolved in 80 mM citric acid overnight at room temperature (protected against light) and stored at 4°C for up to 2 weeks. Before being administered to the mice, dissolved dasatinib was diluted in 80 mM citric buffer (pH 3) to 2.5 mg/mL. (A) TPC-1 (top) and K2 (bottom) cells carrying luciferase were inoculated in situ into the right thyroid lobe of Ncr-nu/nu mice. Dasatinib (15 mg/kg) was delivered by oral gavage either once (QD) or twice (BID) a day in two treatment plans. Average tumor volumes (mm3) were calculated at the end of treatment cycle, plotted in Scatter plots, and t-test was performed using Prism 5.0. (B) Mice were inoculated with TPC-1 or K2 cells. After confirmation the tumor growth in mice by luciferase bioluminescent imaging, mice were treated with 15 mg/kg dasatinib once at 0 h and tumors were harvested at 1, 3, or 24 hr. Phosphorylations of Src and FAK at Tyr 861 site were detected on Western blots. Total Src and total FAK were used as loading controls. (C) Same as in (B) except mice were treated with 15 mg/kg dasatinib twice (12 h apart) and protein extracts were prepared from tumor after 2nd treatment at 1, 3, 6, or 12 hr. The 2nd and the 3rd lanes of vehicle control were from the same mouse and total of 2 tumors from vehicle was shown here.
