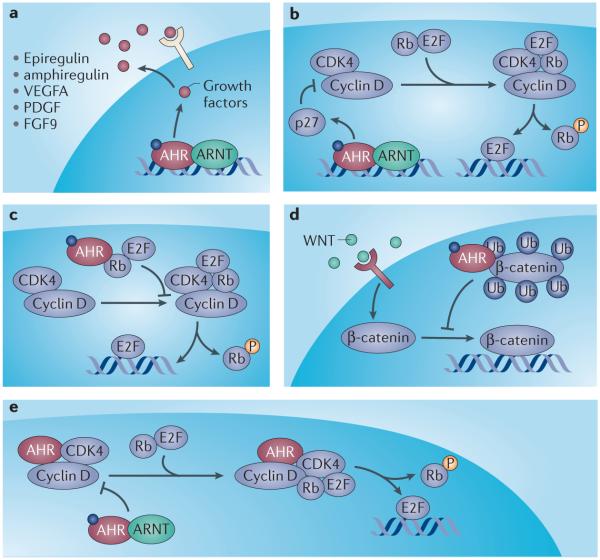
Figure 3. Proposed mechanisms of cell cycle modulation by the AHR.
Multiple mechanisms are proposed to account for the pro-/anti-proliferative action of AHR agonists observed with tumor cells in vitro. 1) Binding to AHR response elements in promoters of their respective genes, AHR acts as a direct transcriptional activator stimulating expression of growth factors epiregulin and amphiregulin. As mitogens, these contribute to the proliferation of tumor cells and may account for AHR agonist-mediated tumor cell expansion. 2) Agonist-activated AHR binds to the promoter of p27 (CDKN1B) enhancing its expression 159, 160. As an inhibitor of cyclin-dependent kinase activity, increased p27 limits phosphorylation of retinoblastoma protein (Rb) thus restricting E2F-dependent gene expression and progression through the cell cycle. 3) Association of AHR with Rb in an agonist-dependent manner attenuates both phosphorylation of Rb and liberation of E2F, resulting in cell cycle arrest and inhibition of proliferation 161. 4) Agonist activation of AHR promotes association with β-catenin and stimulation of a previously unrecognized ubiquitin ligase function of AHR 45, 67. Ubiquitination of β-catenin in an AHR-dependent fashion promotes proteosomal degradation, restricting cell cycle-dependent gene expression and proliferation. 5) In the absence of ligand, AHR forms a complex with cyclin D and the cyclin-dependent kinases CDK4/6, suppressing phosphorylation of Rb and subsequent E2F-mediated gene expression to promote cell cycle arrest 162. Exposure to AHR agonist favors the dissociation of the AHR/cyclinD/CDK complex to permit cell cycle progression and tumor cell proliferation. The contradictory nature of these mechanisms may reflect cell-type/culture-dependent differences and emphasize the need to investigate the effect of AHR ligands in the context of in vivo proliferation models.