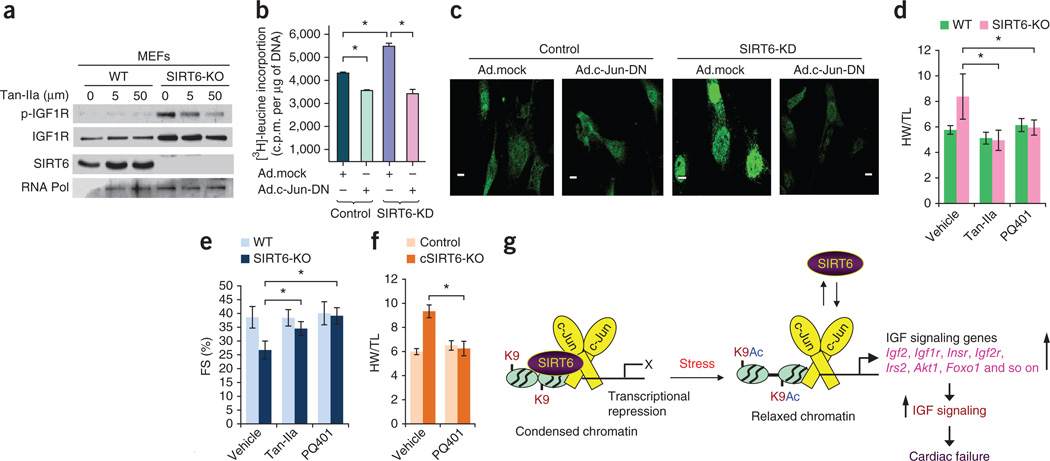
Figure 6.
Inhibition of c-Jun or IGF signaling blocks hypertrophy of SIRT6-deficient hearts. (a) Western blots showing effects of the indicated concentrations of the AP-1 inhibitor Tan-IIa on IGF1R and p-IGF1R abundance in WT and SIRT6 knockout MEFs. (b) [3H]-leucine incorporation into total cellular protein in control and SIRT6 knockdown neonatal rat cardiomyocytes infected with control (Ad.mock) or c-Jun dominant-negative (Ad.c-Jun-DN) adenovirus vectors. Data are presented as the mean ± s.d. n = 6–8 independent experiments. *P < 0.001 (Student’s t test). (c) Confocal imaging of ANF in the same group of cardiomyocytes as in b. Scale bars, 10 µm. (d,e) HW/TL ratio (d) and fractional shortening (FS) (e) of WT and SIRT6 knockout mice injected with vehicle, the AP-1 inhibitor Tan-IIa or the IGF1R inhibitor PQ401 for 2 weeks. n = 6–9 mice per group. Data are presented as the mean ± s.d. *P < 0.001 (ANOVA). (f) HW/TL ratio of control (Sirt6flox/flox) and cardiac-specific SIRT6 knockout mice injected with vehicle or the IGF1R inhibitor PQ401 for 2 weeks. n = 6 mice per group. Data are presented as the mean ± s.d. *P < 0.001 (Student’s t test). (g) Under normal conditions, SIRT6 inhibits the expression of IGF signaling–related genes by deacetylating histones and repressing c-Jun activity, thereby restraining IGF signaling. Under pathological stress, cardiac SIRT6 expression is reduced, leading to increased acetylation of H3K9 (K9Ac) at the promoters of IGF signaling genes and c-Jun–mediated transcriptional activation. Increased expression of multiple IGF-Akt signaling–related genes leads to the development of cardiac hypertrophy, fibrosis and heart failure.