Figure 3. Analysis of the reactions catalysed by module 5.
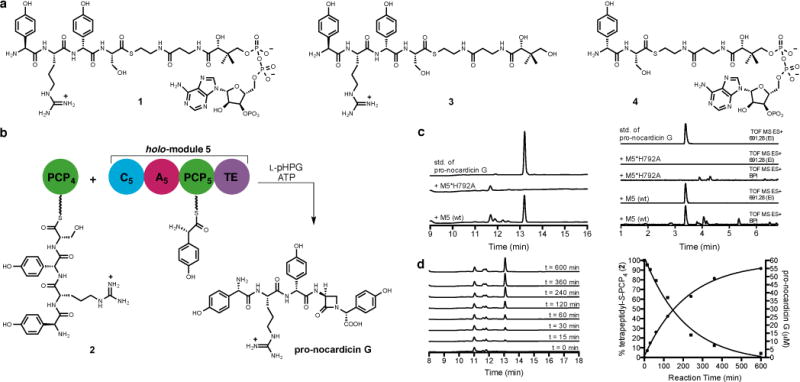
a, Substrates used in this study. b, Incubation of tetrapeptidyl-S-PCP4 2 with holo-module 5, ATP and L-pHPG produced β-lactam containing pro-nocardicin G. c, Left: HPLC traces of products obtained after incubation of tetrapeptidyl-S-PCP4 2 and indicated construct, ATP and L-pHPG. Pro-nocardicin G was observed in the wild-type reaction [+M5(wt)] but not in the mutant (+M5*H792A), verified by comparison to synthetic standard (top trace). Right: LC-MS traces of products obtained after incubation of tetrapeptidyl-S-PCP4 2 and holo-module 5, ATP and L-pHPG. Pro-nocardicin G was observed in the wild-type reaction [+M5(wt)] but not in the mutant (+M5*H792A), verified by comparison to synthetic standard (top trace). BPI = base peak ion, TOF = time of flight, ES = electrospray ionization, EI = extracted ion. Calculated exact mass of pro-nocardicin G = 691.2835 [M+H]+. d, Left: Time-course study of tetrapeptidyl-S-PCP4 2 and holo-module 5 supplemented with L-pHPG and ATP. Appearance of pro-nocardicin G was analyzed by HPLC. Right: Plots of pro-nocardicin G production (HPLC) and the corresponding conversion of 2 to the unloaded holo-PCP4, (ESI-MS). Less than 10% hydrolysis of 2 was observed during the 10-h experiment.
