Figure 4. Proposed β-lactam formation mechanism.
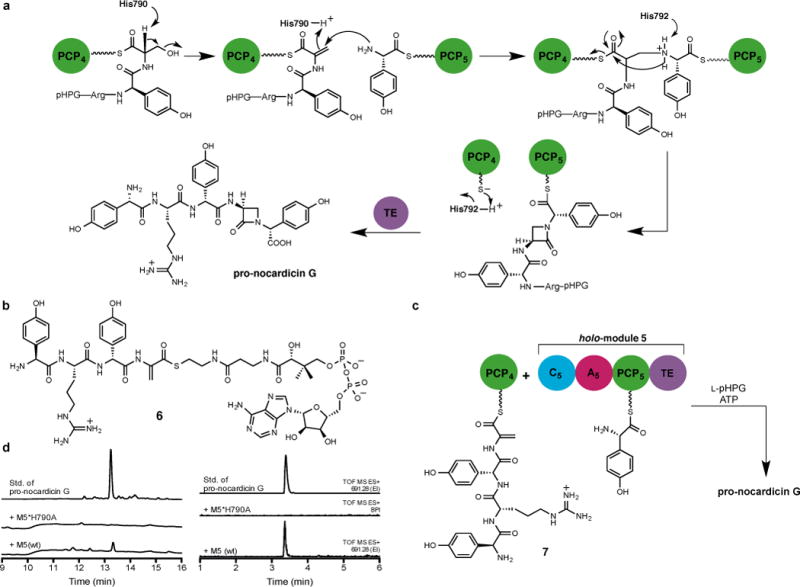
a, Proposed mechanism of β-lactam formation in C5. Tentative catalytic roles of histidine residues are indicated. b, Substrate used in this study. c, Incubation of dehydroalanyl tetrapeptidyl-S-PCP4 7 with holo-module 5, ATP and L-pHPG gave pro-nocardicin G. d, Left: HPLC traces of products obtained after incubation of dehydroalanyl tetrapeptidyl-S-PCP4 7 and indicated holo-module 5, ATP and L-pHPG. Pro-nocardicin G was observed in the wild-type reaction [+M5(wt)] but not in the mutant (+M5*H790A), verified by comparison to synthetic standard (top trace). Right: LC-MS traces of products obtained after incubation of dehydroalanyl tetrapeptidyl-S-PCP4 7 and indicated holo-module 5 construct, ATP and L-pHPG. Pro-nocardicin G was observed in the wild-type reaction [+M5(wt)] but not in the mutant (+M5*H790A), verified by comparison to synthetic standard (top trace). TOF = time of flight, ES = electrospray ionization, EI = extracted ion.
