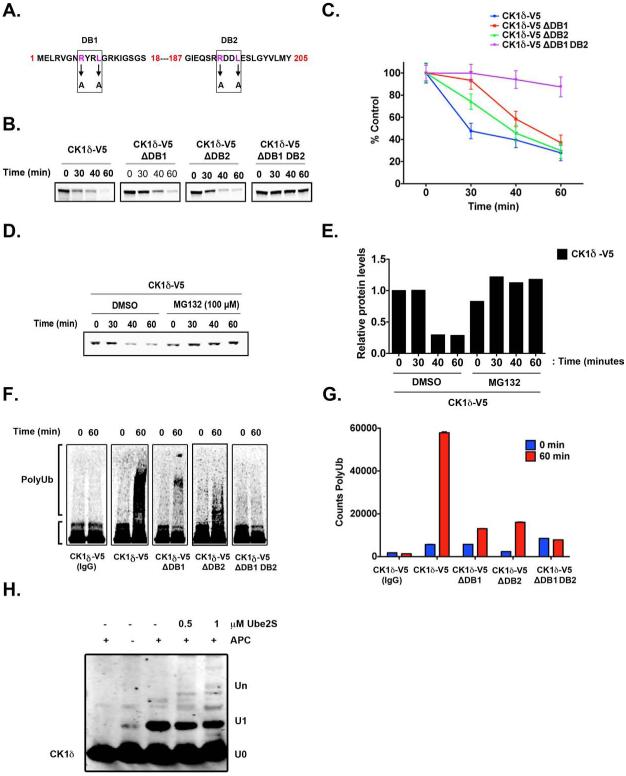
Figure 4. APC/CCdh1 induces CK1δ Ubiquitination and Degradation in vitro.
(A) Two destruction boxes (D-boxes) in human CK1δ, DB1 and DB2, were mutated by substituting alanine (A) for the corresponding arginine (R) and leucine (L) residues. (B-C) Both D-boxes in CK1δ are required for proteolysis. In vitro degradation assay showing 35S-labeled wild-type CK1δ-V5, DB1 mutant (CK1δ-V5 ΔDB1), DB2 mutant (CK1δ-V5 ΔDB2), and DB1 DB2 double-mutant (CK1δ-V5 ΔDB1 DB2) after incubation in extracts prepared from HeLa cells in G1. Samples collected at the indicated time points were analyzed by autoradiography. (C) The quantification of (B); protein levels were measured in three separate experiments using Quantity One image analysis software (Bio-Rad). An unpaired t test was performed, and a p-value of 0.01 was obtained. (D) Autoradiogram showing in vitro degradation of 35S-labeled wild-type CK1δ-V5 in HeLa cell extracts at G1, in the presence or absence of the proteasome 26S inhibitor MG132 (100 μM). (E) Quantification of CK1δ-V5 from (D). (F-G) Both D-boxes in CK1δ are required for efficient ubiquitination. Autoradiogram of 35S-labeled wild-type CK1δ-V5, ΔDB1, ΔDB2, and ΔDB1 DB2 mutants after in vitro ubiquitination by anti-Cdc27 immunoprecipitates from HeLa cell extracts at G1. (G) The extent of polyubiquitination was quantified for the entire lane above the inputs by using Quantity One image analysis software. From three separate experiments, an unpaired t test was performed, and a p-value of 0.005 was obtained. Results shown are the averages of three independent experiments and are represented as the mean ± SEM. (H) Purified CK1δ and immunoprecipitated APC/C were incubated together in vitro, and the extent of ubiquitination was determined after SDS-PAGE and anti-CK1δ autoradiography.