Table 2. Synthesis of Diverse Polycyclic Heterocycles Using the Catalytic C–H Alkylation.
| Entry | Substrate | Product | yield(%)a |
|---|---|---|---|
|
|
||
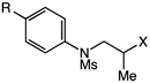
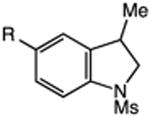
| 1b | 27: X = Br, R = H | 28: R = H | 54 |
| 2 | 29: X = I, R = H | 82 | |
| 3 | 30: X = I, R = CF3 | 31: R = CF3 | 70 |
| 4 | 32: X = I, R = OMe | 33: R = OMe | 66 |
| 5 | 34: X = I, R = B(pin) | 35: R = B(pin) | 72 |
| 6 | 36: X = I, R = C(O)Me | 37: R = C(O)Me | 77 |
| 7 |
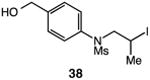
|
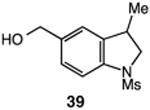
|
57 |
| 8 |
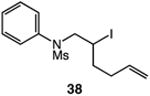
|
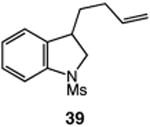
|
80 |
|
|
||
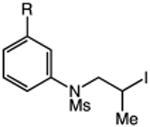
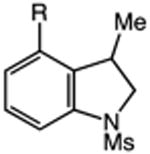
| 9 | 40: R = CF3 | 41: R = CF3 | 81: (2.4:1 o:p) |
| 10 | 42: R = Me | 43: R = Me | 91c(2.0:1 o:p) |
| 11 |
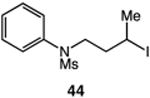
|
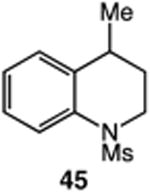
|
57 |
| 12 |
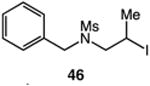
|
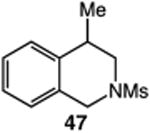
|
61 |
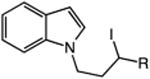
|
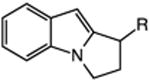
|
||
| 13 | 48: R = H | 49: R = H | 51c |
| 14 | 50: R = Me | 51: R = Me | 71c |
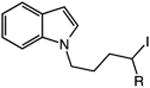
|
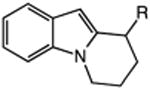
|
||
| 15 | 52: R = H | 53: R = H | 70c |
| 16 | 54: R = Me | 55: R = Me | 90c |
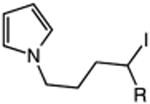
|
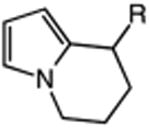
|
||
| 17 | 56: R = H | 57: R = H | 64c |
| 18 | 58: R = Me | 59: R=Me | 95c |
All reactions were performed with [substrate]0 = 0.5 M in dioxane at 100 °C with 10 mol % Pd(PPh3)4 and 2 equiv K3PO4 as base.
Isolated yields.
The reaction was performed at 130 °C in PhtBu.
Calculated by 1H NMR spectroscopy of crude reaction mixtures using an internal standard.
