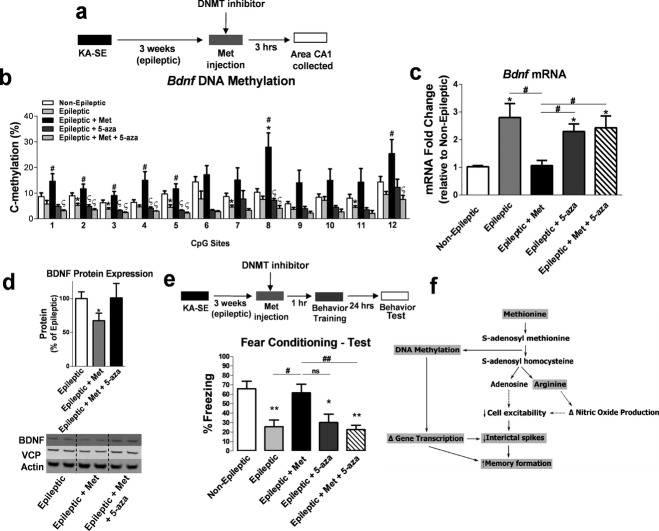
Figure 6.
DNA methyltransferases (DNMT) inhibition prevents the effect of methyl supplementation on hippocampal brain-derived neurotrophic factor (Bdnf) DNA methylation, Bdnf expression and memory formation. (a) Diagram of experimental setup. (b) The increase in BdnfDNA methylation due to methyl supplementation is blocked by DNMT inhibition (F4,18 = 6.93, P < 0.01, n = 3–6, one-way analysis of variance [ANOVA] with post hoc test, *significance relative to non-epileptic group; # significance relative to epileptic group; §significance relative to epileptic + Met group). (c) DNMT inhibition attenuated the effect of methyl supplementation with Met on BdnfmRNA levels in the epileptic hippocampus (F4,24 = 11.96, P < 0.001, n = 5–8, one-way ANOVA with post hoc test, *significance relative to non-epileptic group; #significance between experimental groups). (d) BDNF protein expression was significantly reduced in Met-treated epileptic animals compared to nontreated epileptic animals. DNMT inhibition attenuated the decrease in BDNF protein expression due to methyl supplementation with Met. Duplicates of each sample were run and normalized to valosin-containing protein (VCP) and beta-actin 4 as within-lane loading controls. Representative blots of samples from each treatment group are shown (F3,14 = 3.84, P < 0.05, n = 5–6, one-way ANOVA with post hoc test, *significance relative to epileptic group). (e) DNMT inhibition blocked Met-induced memory enhancement (F4,31 = 7.81, P < 0.001, n = 7–8, one-way ANOVA with post hoc test, *significance relative to non-epileptic group; #significance between experimental groups). Error bars are SEM. (f) Potential mechanism of the effect of methionine treatment on memory restoration in epileptic animals. Shaded words represent the molecular mechanisms demonstrated in the present study. Unshaded words and dotted lines are potential pathways that could also be involved in the memory restoration process with methionine. Thus, methionine can be rapidly converted to S-adenosyl methionine (SAM), the universal methyl donor in the brain. SAM donates a CH3 group that leads to increased DNA methylation and gene transcription changes in the epileptic hippocampus. Once SAM donates a methyl group, it is converted to S-adenosyl-l-homocysteine (SAH), which leads to the production of adenosine. Adenosine production can lead to decreases in cell excitability that could subsequently decrease interictal spike activity in the epileptic hippocampus. Arginine is also a downstream by product of SAH hydrolysis that can lead to changes in nitric oxide production and can affect cell excitability.