Figure 4.
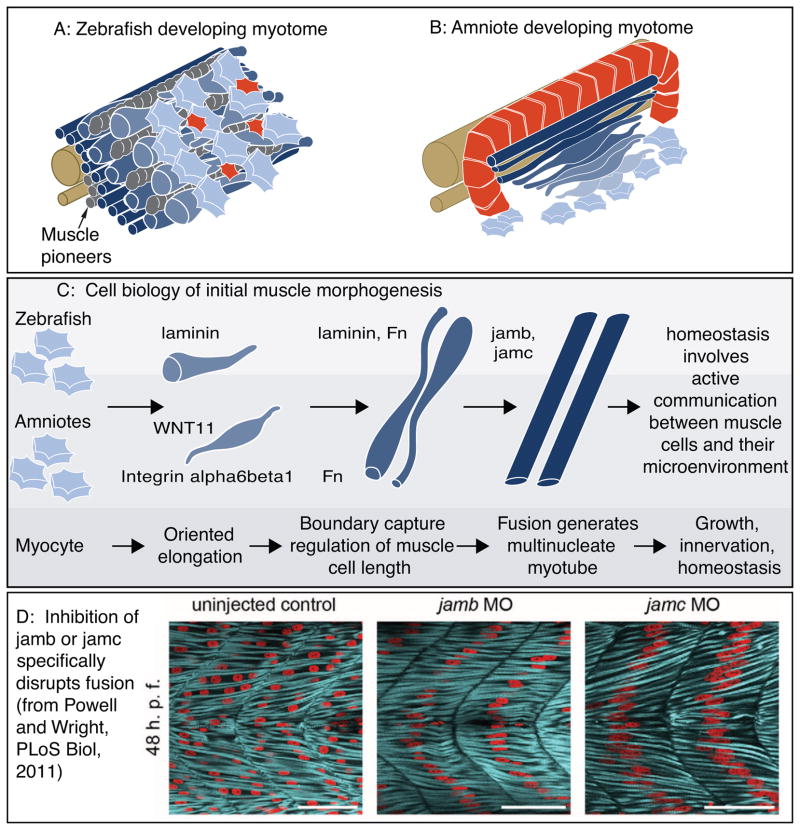
Cartoon of muscle morphogenesis in chick and zebrafish embryos. A: Developing zebrafish myotome. Slow-twitch fibers (gray) have migrated partway through the fast-twitch domain. Fast-twitch muscle cells (dark blue) medial to the slow-twitch fibers are long. Fast-twitch cells (medium blue) directly intermingled with migrating slow-twitch fibers are elongating. Lateral to the slow-twitch fibers are short muscle precursor cells (light blue) extending protrusions in all directions and the external cell layer (red). B: Developing chick myotome. Note that short myoblasts extend protrusions in all directions, but once elongation has begun protrusions are extended only in the direction of elongation. C: More detailed view of the initial stages of muscle fiber morphogenesis that are similar in amniotes and zebrafish. Short precursor cells extend protrusions in all directions. Once cells begin elongating, they do so in an oriented fashion. Adhesion to laminin is required for oriented myocyte elongation in both species, WNT11 is required for oriented elongation in chick. Boundary capture involves the cessation of muscle cell elongation and regulates muscle cell length. Fn regulates myotomal muscle cell length in both zebrafish and chick, and laminin has been shown to play a role in boundary capture in the zebrafish system. D: Genes required for muscle fusion in zebrafish. To date, the only proteins whose disruption leads to a specific defect in fusion (with all other aspects of muscle morphogenesis appearing normal) are jamb and jamc. Once fusion has occurred, myofibrillogenesis, growth, innervation, and homeostasis all require adhesion to the ECM.