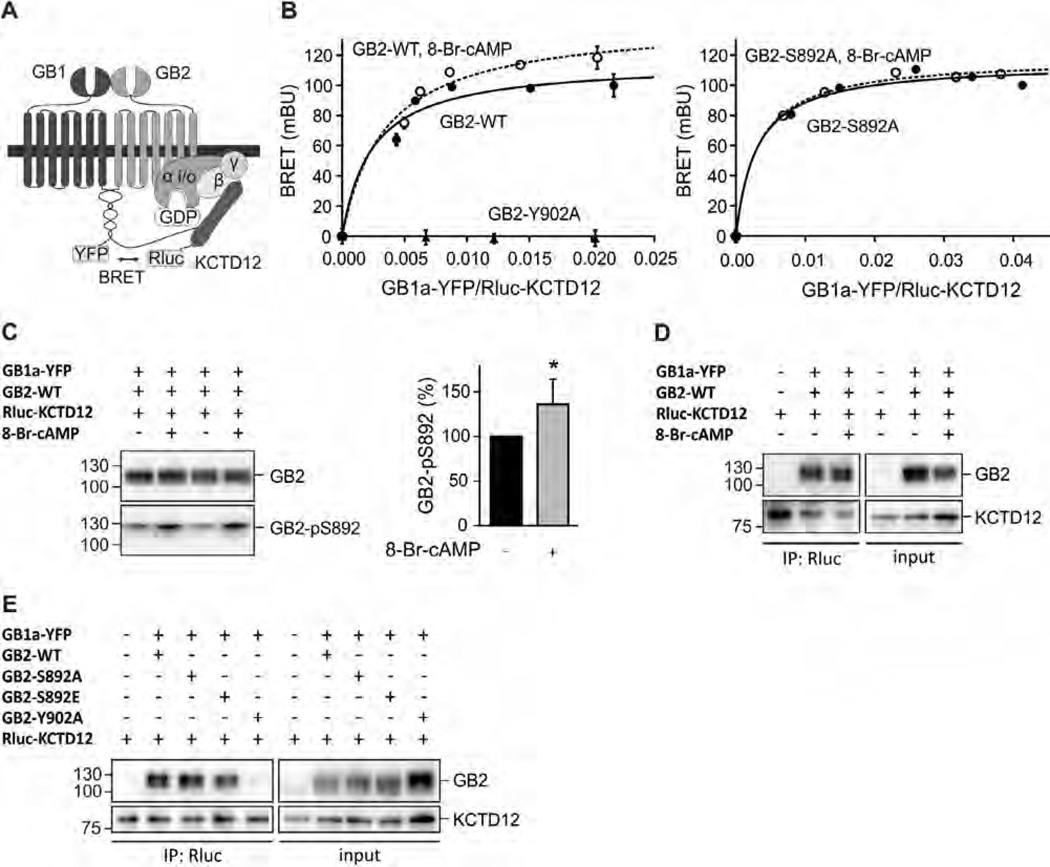
Fig. 2.
S892 phosphorylation rearranges the receptor/KCTD12 complex. (A) Scheme of the BRET Rluc-KCTD12 donor and GB1a-YFP acceptor pair. (B) BRET donor saturation curves from HEK293T cells expressing fixed amounts of GB2-WT, GB2-Y902A (both left) or GB2-S892A (right) together with Rluc-KCTD12 and increasing amounts of GB1a-YFP. Cells were pre-incubated with 8-Br-cAMP(1 mM, 30 min; open circles) to activate PKA. BRET is expressed as milli BRET units (mBU) determined as net BRET × 1000. Data points are means ± SD of technical quadruplicates of a representative experiment, n = 4–7. (C) Western blot of lysates of HEK293T cells expressing GB1a-YFP, GB2-WT and Rluc-KCTD12 in the presence and absence of 8-Br-cAMP(1 mM, 30 min pre-incubation). Activation of PKA does not affect the total amount of GB2 but increases phosphorylation at S892 (GB2-pS892). GB2 runs as two bands on SDS–PAGE, likely due to differences in posttranslational modification. The higher molecular weight band appears to be more heavily phosphorylated at S892. Blots are representative of six independent experiments. Bar graph summarizing the amount of S892 phosphorylation normalized to the amount of GB2 on the same blot (GB2-pS892 in %). Data are means ± SD, n = 6. *, p < 0.05; t test. (D) Co-immunoprecipitation of GABAB receptors with KCTD12 from HEK293T cells expressing GB1a-YFP, GB2-WT and Rluc-KCTD1 2 in the presence and absence of 8-Br-cAMP(1 mM, 30 min pre-incubation). Equivalent amounts of GABAB receptors were co-precipitated. Blots are representative of five independent experiments. (E) Co-immunoprecipitation of GABAB receptors with KCTD12 from HEK293T cells expressing GB1a-YFP, GB2 (WT, S892A, S892E or Y902A) and Rluc-KCTD12. Y902A does not bind KCTD12 and was used as a negative control. Blots are representative of three independent experiments.