Fig. 1.
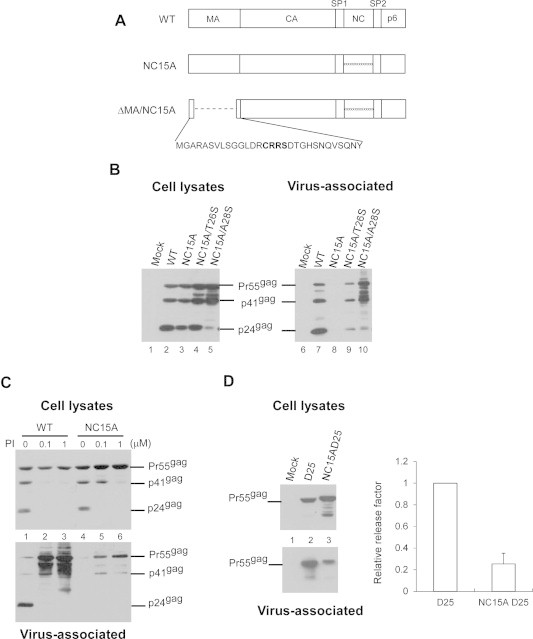
Assembly and processing of HIV-1 mutants containing alanine replacement of all NC basic residues. (A) Schematic representations of wild-type (WT) and recombinant HIV-1 mutants. Indicated are the HIV-1 Gag protein domains MA (matrix), CA (capsid), NC (nucleocapsid), p6, and two spacer peptides SP1 and SP2. “xxxxxxxxxx” denotes all NC basic residues replaced with alanines. ΔMA contains a replacement of 106 codons by 4 codons (in bold face), with myristylation and MA/CA cleavage signal retained. (B–D) Suppression of PR activity alleviates the assembly defect incurred by positive nucleocapsid charge elimination. (B) 293T cells were transfected with 20 μg of designated plasmid. T26S and A28S are PR point mutations triggering 4-fold and 50-fold reductions in PR activity, respectively [38]. At 48 h post-transfection, culture supernatants and cells were collected and prepared for protein analysis as described in Section 2. Viral pellet samples corresponding to 50% of total samples (lanes 6–10) and cell lysate samples corresponding to 5% of total samples (lanes 1–5) were fractionated using 10% SDS–PAGE and electroblotted onto nitrocellulose filters. HIV-1 Gag proteins were probed with a mouse monoclonal antibody directed at p24CA. Positions of HIV Gag proteins Pr55, p41, and p24 are indicated. (C) 293T cells were transfected with wt or mutant plasmids. At 4 h post-transfection, cells were replated on three dish plates and either left untreated (lanes 1 and 4) or treated with the HIV-1 protease inhibitor (PI) saquinavir at concentrations of 0.1 μM (lanes 2 and 5) or 1.0 μM (lanes 3 and 6). At 48–72 h post-transfection, cells and culture supernatant were collected, prepared, and subjected to Western immunoblot analysis. (D) 293T cells were transfected with the PR-inactivated version (D25) of NC15A. At 48–72 h post-transfection, culture supernatants and cells were collected and subjected to Western immunoblotting.
