Abstract
Interleukin 17A (IL-17) production by peripheral blood neutrophils was examined in patients with fungal keratitis and in uninfected individuals in southern India, which has high levels of airborne Aspergillus and Fusarium conidia. Il17a gene expression and intracellular IL-17 were detected in all groups, although levels were significantly elevated in neutrophils from patients with keratitis. There were no significant differences in plasma IL-17 and IL-23 between patients with keratitis and uninfected individuals; however, combined data from all groups showed a correlation between the percentage IL-17 producing neutrophils and plasma IL-23, and between plasma IL-17 and IL-6 and IL-23.
Keywords: neutrophil, IL-17, IL-23, fungal infection, Aspergillus, Fusarium, corneal ulcer, keratitis
Individuals with mutations resulting in impaired interleukin 17A (IL-17) production exhibit an increased susceptibility to mucocutaneous candidiasis [1, 2]. Although CD4+ Th17 cells and innate lymphoid cells have been cited as the predominant source of IL-17 in microbial infections and in autoimmunity [3], neutrophils have also been reported as a source of IL-17 in human psoriatic lesions and in cutaneous T cell lymphoma [4, 5].
IL-17 gene expression is significantly elevated in corneal ulcers caused by Fusarium and Aspergillus in which neutrophils comprise >90% of infiltrating cells in the ulcers [6]. Furthermore, >70% of total peripheral blood neutrophils from healthy individuals in the United States constitutively express receptors for interleukin 6 (IL-6) and interleukin 23 (IL-23) and can produce IL-17 following stimulation with these cytokines [7].
As airborne spores induce IL-23 production and Th17 cells [8], and healthy individuals have T cell responses to Aspergillus [9], we examined whether IL-17–producing neutrophils are generated in individuals exposed to high levels of airborne spores, we examined IL-17–expressing neutrophils in patients with fungal keratitis and healthy cohorts in an agricultural region of southern India.
METHODS
Peripheral Blood Neutrophils
Peripheral blood was suspended in 3% dextran, and plasma was separated by centrifugation. Neutrophils were isolated using Lymphoprep and suspended in complete Roswell Park Memorial Institute medium (all reagents were from Sigma, St. Louis, MO). Giemsa staining showed >95% neutrophils.
Flow Cytometry
Neutrophils were incubated with Fc blocking reagent (eBioscience) at 4°C for 30 minutes. For intracellular staining, cells were fixed with 4% paraformaldehyde, incubated with 1 × permeabilization buffer (eBioscience), and incubated for 1 hour with rabbit serum (Vector laboratories) to block Fc receptors. Neutrophils were then incubated with anti–IL-17 or isotype control (eBiosciences) and analyzed on a BD FACS Calibur platform, using CellQuest software and FlowJo (Tree Star) software. Gates and quadrants were determined using the isotype controls.
Immunofluorescence
For direct immunofluorescent staining, intracellular IL-17 was detected in neutrophils after incubation with antibodies as described above. Peripheral blood neutrophils were centrifuged onto charged microscope slides, using a Cytospin, and Vectashield medium with DAPI was added (Vector Labs). Infected corneal ulcer material was spread onto microscope slides as we described previously [6]. IL-17–positive and IL-17–negative cells were detected by fluorescence microscopy.
Quantitative Polymerase Chain Reaction (PCR)
Quantitative PCR for detection of IL-17 was performed as described previously [6, 10], with normalization for β-actin expression, and 2−ΔΔct was calculated.
Plasma Cytokines
Cytokines in plasma were analyzed by an enzyme-linked immunosorbent assay according to the manufacturer's protocol (R&D).
Statistical Analysis
Statistical analyses were performed by 1-way analysis of variance and the Bonferroni multiple comparisons test or by Spearman rank correlation coefficient analysis (Prism, GraphPad Software V5.0, San Diego, CA). A P value of < .05 was considered statistically significant.
RESULTS
Study Populations
Informed consent was obtained from all subjects in accordance with the Declaration of Helsinki and the Institutional Review Board of the Aravind Hospitals of Tamil Nadu. A total of 128 subjects were enrolled and assigned to one of 3 groups: patients with fungal keratitis, cohorts with high exposure to airborne conidia but no disease, and healthy controls who work in a facility with filtered air. Of the 93 patients with corneal ulcers, 59 were caused by Fusarium, and 34 were caused by A. flavus. The mean age (±SD) of each group was 45.75 ± 13.10 years (patients), 36.73 ± 12.56 years (cohorts), and 31.78 ± 9.45 years (controls). Patients with fungal keratitis had no other disease symptoms.
Intracellular IL-17 in Peripheral Blood and Corneal Neutrophils
A recent study showed that peripheral blood neutrophils from healthy individuals in the United States do not express or produce IL-17 [7]. We therefore examined IL-17 expression in peripheral blood neutrophils from patients with fungal keratitis and from healthy individuals exposed to high levels of airborne spores.
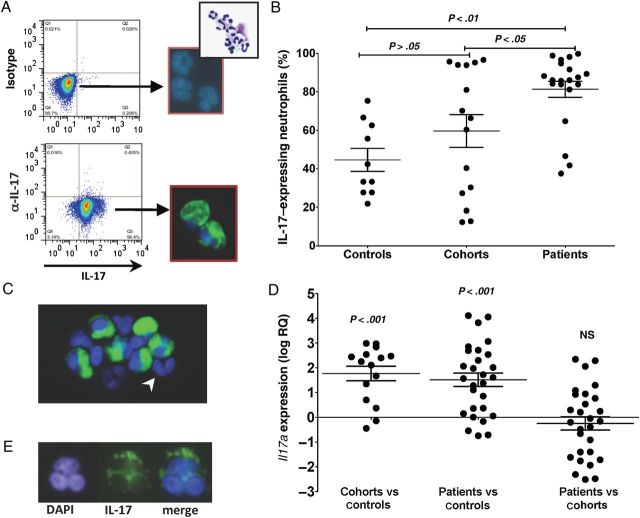
As shown in Figure 1A, a distinct population of IL-17–producing neutrophils was identified by flow cytometry and confirmed by fluorescence microscopy. Consistent with our prior findings comparing Fusarium and Aspergillus responses [6], there was no significant difference in the percentage of IL-17–producing neutrophils between Fusarium- and Aspergillus-infected patients (data not shown); therefore, we pooled data from these patients for comparison with cohort and control groups. The percentage of IL-17–producing neutrophils in patients with fungal keratitis was significantly higher than cohorts and controls (mean ± SD, 80.9% ± 19.2% for patients vs 59.6% ±31.9% for cohorts and 48.1 ± 19.7 for controls; P < .05, for patients vs cohorts and patients vs controls; Figure 1B). In contrast, there was no significant difference in the percentage of IL-17–producing neutrophils between cohorts and regional controls. IL-17–negative neutrophils were detected in the same field as positive cells (Figure 1C), indicating that the intracellular IL-17 reactivity was specific.
Figure 1.
Interleukin 17A (IL-17)–expressing peripheral blood neutrophils. Intracellular IL-17 (A and B) and Il17a gene expression (C and D) in peripheral blood neutrophils from patients with fungal keratitis, cohorts with high exposure to airborne conidia but no disease, and regional healthy controls. A, Representative flow cytometry profiles showing peripheral blood neutrophils from a patient with fungal keratitis after intracellular staining for IL-17. Neutrophils showing intracellular IL-17 by fluorescence microscopy (multilobed nuclei are visible after DAPI staining, and a representative hematoxylin-eosin stain shows a highly purified population of neutrophils). B, Percentage of IL-17–expressing neutrophils in peripheral blood of patients with keratitis caused by Aspergillus or Fusarium organisms (combined) and from cohorts with high exposure to airborne conidia but no disease, and from regional controls. Data are mean ± standard error of the mean (SEM), and data points represent individuals. C, IL-17–negative neutrophils in the same field as IL-17–positive cells (arrowhead). D, Il17a transcripts in peripheral blood neutrophils normalized to β-actin expression, calculated by the 2−ΔΔct method as described in Methods. Data are s mean ± SEM of the ratio of each patient to the mean of cohorts (patients vs cohorts) or to the mean of regional controls (patients vs controls). Data also show the ratio of each cohort to the mean of regional controls (cohorts vs controls). E, Representative neutrophils from a corneal ulcer showing intracellular IL-17. Original magnification ×600 for images showing fluorescence staining and ×200 for images showing hematoxylin-eosin staining. Abbreviation: NS, not significant.
To examine IL-17 gene expression, RNA was extracted from peripheral blood neutrophils, and Il17a expression was assessed by quantitative PCR. Il17a was detected in individuals from each group; however, the ΔΔct scores were significantly higher in patients and cohorts, compared with regional controls, whereas there were no significant differences between cohorts and patients (Figure 1D). IL-17 was also detected in neutrophils from corneal ulcers of patients with fungal keratitis (Figure 1E).
Overall, these data show that peripheral blood neutrophils from individuals in each group express IL-17 transcripts and protein, although expression was statistically higher in the patient group.
Plasma IL-6, IL-23, and IL-17
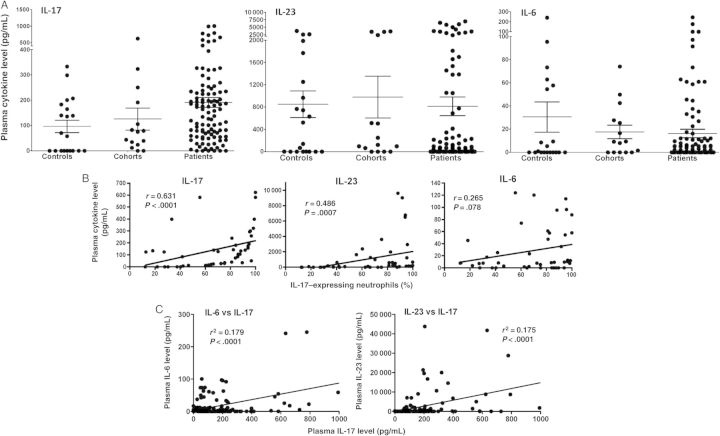
As IL-6 and IL-23 induce IL-17 gene expression in human and murine neutrophils [7], we examined IL-6, IL-23, and IL-17 levels in the plasma of patients with fungal keratitis, compared with cohorts and regional controls. Figure 2A shows that the mean concentration (±SD) of IL-17 in patients was 190.8 ±200.3 pg/mL, 125.6 ± 167.1 pg/mL in cohorts, and 96.4 ±108.3 pg/mL in regional controls. Although the mean value of IL-17 was higher in patient samples, there were no significant differences among any of the groups. Similarly, there were no significant differences among the groups in plasma IL-23 and IL-6 levels (Figure 2A).
Figure 2.
Plasma levels of interleukin 6 (IL-6), interleukin 23 (IL-23), and interleukin 17A (IL-17). A, Plasma was collected from patients with fungal keratitis (n = 93), cohorts (n = 15), and regional controls (n = 20), and cytokine levels were measured by enzyme-linked immunosorbent assay. Data are mean±standard error of the mean, and data points represent individuals. B, Spearman rank correlation analyses of 45 individuals (patients, cohorts, and regional controls combined) comparing the percentage of IL-17–expressing peripheral blood neutrophils with plasma levels of IL-17, IL-23, and IL-6 in each individual. C, Correlation between plasma levels of IL-17 and plasma levels of IL-6 and IL-23 (n = 121).
To determine whether there is a correlation between plasma levels of IL-17, IL-23, or IL-6 and IL-17–producing neutrophils, we performed Spearman correlation coefficient analyses on all 45 individuals for whom we had percentage neutrophil data (combining patients, cohorts, and controls) with their corresponding plasma cytokine concentrations. We found a correlation between the IL-17–producing neutrophils and the concentration of plasma IL-17 (P < .0001) and plasma IL-23 (P < .001); however, there was no correlation with the concentration of IL-6 (P > .05; Figure 2B). However, there was a correlation between plasma IL-17 and plasma IL-6 and IL-23 concentrations (Figure 2C).
DISCUSSION
In India and other developing nations, the major risk factors for corneal ulcers caused by Aspergillus and Fusarium molds are agricultural work and corneal injury [11]. Fungal infections stimulate production of antimicrobial peptides in tears that likely contribute to host defense [12]; however, we reported that >90% of the total cells in corneal ulcer scrapings examined within a week of infection are neutrophils and that Il17a gene expression by PCR analysis is elevated by 10 000-fold, compared with normal donor corneas [6]. In the current study, we examined IL-17 RNA and protein expression in highly purified populations of peripheral blood neutrophils. We also compared patients with fungal keratitis with uninfected cohorts (relatives and neighbors from the same village) and with individuals (controls) in Madurai. As exposure to molds is primarily by inhalation of airborne conidia, it seemed likely that patients and cohorts would have similar levels of plasma IL-17 and IL-17–producing neutrophils and that these levels would be higher than those in individuals not working directly in agricultural regions (controls).
Surprisingly, we found that patients with fungal keratitis had a significantly higher percentage of IL-17–producing neutrophils and IL-17 gene expression than cohorts, which may be a result of corneal inflammation and cytokine production in response to growing hyphae. As predicted, the patient and cohort groups had a higher percentage of IL-17–expressing neutrophils than the control group. The presence of IL-17–producing neutrophils in healthy individuals also supports the concept that these cells are recruited to, rather than generated in infected corneas.
Although there were no differences in total plasma levels of IL-6, IL-17, and IL-23 among the study groups, when data were combined for all individuals and correlation analyses were performed, we found a significant correlation between the percentage of IL-17–positive neutrophils and systemic levels of IL-17 and IL-23 and a significant correlation between plasma levels of IL-17 and plasma levels of IL-6 and IL-23. These findings are consistent with but not evidence of a role for these cytokines in IL-17 production. Further, although recombinant IL-6 and IL-23 are sufficient to induce IL-17 expression by neutrophils [7], higher concentrations than found in the plasma are required, suggesting that that additional cytokines are also involved.
Patients with autoimmune diseases such as anti–neutrophil cytoplasmic antibody–associated vasculitis and ankylosing spondylitis also have very high serum levels of IL-23 (3000–6000 pg/mL), compared with undetectable levels in normal individuals [13, 14], and patients with Graves disease of the orbit have elevated IL-23 levels (>800 pg/mL), compared with normal individuals [15].
Approximately 80% of peripheral blood neutrophils in healthy individuals in the United States constitutively express IL-6 and IL-23 receptors, and IL-17 gene expression is induced following incubation with recombinant IL-6 and IL-23 [7]. These cytokines also stimulate expression of a functional IL-17 receptor on neutrophils, and autocrine IL-17/IL-17 receptor activation mediates increased production of reactive oxygen species and fungal killing [7]. Therefore, IL-17–producing neutrophils generated by long-term exposure to airborne conidia may limit the ability of Aspergillus and Fusarium to cause active infection, although they may also contribute to tissue damage. Future studies will examine whether IL-17–producing neutrophils generated during infection have these properties and explore the effect of blocking IL-17 receptor activation as an approach to limit tissue damage.
Notes
Financial support. This work was supported by the Indian Council of Medical Research (grant to P. L.) and the Alcon Research Institute (to E. P.).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.de Beaucoudrey L, Puel A, Filipe-Santos O, et al. Mutations in STAT3 and IL12RB1 impair the development of human IL-17-producing T cells. J Exp Med. 2008;205:1543–50. doi: 10.1084/jem.20080321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Puel A, Doffinger R, Natividad A, et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J Exp Med. 2010;207:291–7. doi: 10.1084/jem.20091983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 4.Fontao L, Brembilla NC, Masouye I, et al. Interleukin-17 expression in neutrophils and Th17 cells in cutaneous T-cell lymphoma associated with neutrophilic infiltrate of the skin. Br J Dermatol. 2011;166:687–9. doi: 10.1111/j.1365-2133.2011.10647.x. [DOI] [PubMed] [Google Scholar]
- 5.Lin AM, Rubin CJ, Khandpur R, et al. Mast cells and neutrophils release IL-17 through extracellular trap formation in psoriasis. J Immunol. 2011;187:490–500. doi: 10.4049/jimmunol.1100123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karthikeyan RS, Leal SM, Jr, Prajna NV, et al. Expression of innate and adaptive immune mediators in human corneal tissue infected with Aspergillus or Fusarium. J Infect Dis. 2011;204:942–50. doi: 10.1093/infdis/jir426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Taylor PR, Roy S, Leal SM, Jr, et al. Activation of neutrophils by autocrine IL-17A-IL-17RC interactions during fungal infection is regulated by IL-6, IL-23, RORgammat and dectin-2. Nat Immunol. 2014;15:143–51. doi: 10.1038/ni.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chamilos G, Ganguly D, Lande R, et al. Generation of IL-23 producing dendritic cells (DCs) by airborne fungi regulates fungal pathogenicity via the induction of T(H)-17 responses. PLoS One. 2010;5:e12955. doi: 10.1371/journal.pone.0012955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaudhary N, Staab JF, Marr KA. Healthy human T-cell responses to Aspergillus fumigatus antigens. PLoS One. 2010;5:e9036. doi: 10.1371/journal.pone.0009036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karthikeyan RS, Priya JL, Leal SM, Jr, et al. Host response and bacterial virulence factor expression in Pseudomonas aeruginosa and Streptococcus pneumoniae corneal ulcers. PLoS One. 2013;8:e64867. doi: 10.1371/journal.pone.0064867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bharathi MJ, Ramakrishnan R, Meenakshi R, Padmavathy S, Shivakumar C, Srinivasan M. Microbial keratitis in South India: influence of risk factors, climate, and geographical variation. Ophthalmic Epidemiol. 2007;14:61–9. doi: 10.1080/09286580601001347. [DOI] [PubMed] [Google Scholar]
- 12.Ananthi S, Venkatesh Prajna N, Lalitha P, Valarnila M, Dharmalingam K. Pathogen induced changes in the protein profile of human tears from Fusarium keratitis patients. PLoS One. 2013;8:e53018. doi: 10.1371/journal.pone.0053018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nogueira E, Hamour S, Sawant D, et al. Serum IL-17 and IL-23 levels and autoantigen-specific Th17 cells are elevated in patients with ANCA-associated vasculitis. Nephrol Dial Transplant. 2010;25:2209–17. doi: 10.1093/ndt/gfp783. [DOI] [PubMed] [Google Scholar]
- 14.Mei Y, Pan F, Gao J, et al. Increased serum IL-17 and IL-23 in the patient with ankylosing spondylitis. Clin Rheumatol. 2011;30:269–73. doi: 10.1007/s10067-010-1647-4. [DOI] [PubMed] [Google Scholar]
- 15.Kim SE, Yoon JS, Kim KH, Lee SY. Increased serum interleukin-17 in Graves’ ophthalmopathy. Graefes Arch Clin Exp Ophthalmol. 2012;250:1521–6. doi: 10.1007/s00417-012-2092-7. [DOI] [PubMed] [Google Scholar]