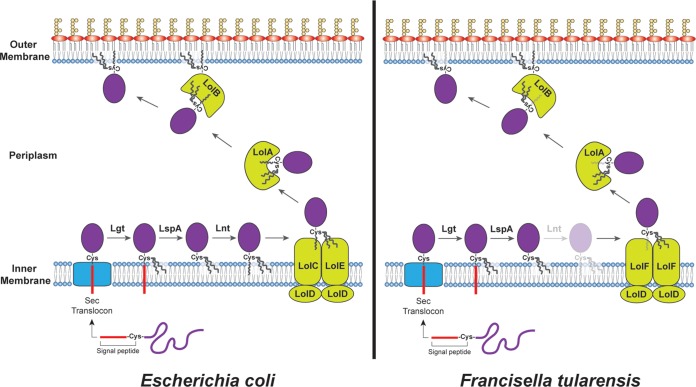
FIG 1.
Models for lipoprotein processing and sorting in Gram-negative bacteria. (Left) The current model for lipoprotein biogenesis in E. coli. The N-terminal signal peptide directs cytoplasmic preprolipoproteins to the Sec complex for translocation to the periplasm. Following passage through the Sec system, Lgt adds a diacylglyceride to the +1 cysteine, and LspA cleaves the signal peptide. Lnt then adds a third acyl chain to the newly available +1 cysteine amino group. The LolCDE transporter complex uses energy from ATP hydrolysis to extract the mature, triacylated lipoprotein from the IM. The LolA chaperone takes the lipoprotein from LolCDE and delivers it to the OM-anchored lipoprotein LolB, which then facilitates insertion of the lipoprotein into the OM. (Right) Lipoprotein biogenesis in F. tularensis, based on the results of LoVullo et al. (5). The Lol transporter complex in Francisella is composed of LolDF instead of LolCDE. The LolDF complex is able to recognize and extract diacylated as well as triacylated lipoproteins. Diacylated lipoproteins would result from loss of Lnt activity (indicated in the figure by increased transparency).